Functional analysis of the Na+,K+/H+ antiporter PeNHX3 from the tree halophyte Populus euphratica in yeast by model-guided mutagenesis
- PMID: 25093858
- PMCID: PMC4122410
- DOI: 10.1371/journal.pone.0104147
Functional analysis of the Na+,K+/H+ antiporter PeNHX3 from the tree halophyte Populus euphratica in yeast by model-guided mutagenesis
Erratum in
-
Correction: Functional Analysis of the Na+,K+/H+ Antiporter PeNHX3 from the Tree Halophyte Populus euphratica in Yeast by Model-Guided Mutagenesis.PLoS One. 2015 Feb 3;10(2):e0117869. doi: 10.1371/journal.pone.0117869. eCollection 2015. PLoS One. 2015. PMID: 25646763 Free PMC article.
Abstract
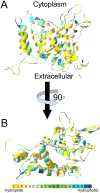
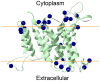
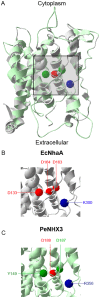
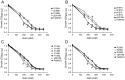
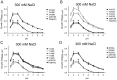
Na+,K+/H+ antiporters are H+-coupled cotransporters that are crucial for cellular homeostasis. Populus euphratica, a well-known tree halophyte, contains six Na+/H+ antiporter genes (PeNHX1-6) that have been shown to function in salt tolerance. However, the catalytic mechanisms governing their ion transport remain largely unknown. Using the crystal structure of the Na+/H+ antiporter from the Escherichia coli (EcNhaA) as a template, we built the three-dimensional structure of PeNHX3 from P. euphratica. The PeNHX3 model displays the typical TM4-TM11 assembly that is critical for ion binding and translocation. The PeNHX3 structure follows the 'positive-inside' rule and exhibits a typical physicochemical property of the transporter proteins. Four conserved residues, including Tyr149, Asn187, Asp188, and Arg356, are indentified in the TM4-TM11 assembly region of PeNHX3. Mutagenesis analysis showed that these reserved residues were essential for the function of PeNHX3: Asn187 and Asp188 (forming a ND motif) controlled ion binding and translocation, and Tyr149 and Arg356 compensated helix dipoles in the TM4-TM11 assembly. PeNHX3 mediated Na+, K+ and Li+ transport in a yeast growth assay. Domain-switch analysis shows that TM11 is crucial to Li+ transport. The novel features of PeNHX3 in ion binding and translocation are discussed.
Conflict of interest statement
Figures



Similar articles
-
Domain-switch analysis of PeNHX3 from Populus euphratica reveals the critical role of the transmembrane domain 11 in Na+ and Li+ transport.J Plant Physiol. 2017 Dec;219:1-11. doi: 10.1016/j.jplph.2017.09.003. Epub 2017 Sep 19. J Plant Physiol. 2017. PMID: 28946051
-
Molecular characterization of putative vacuolar NHX-type Na(+)/H(+) exchanger genes from the salt-resistant tree Populus euphratica.Physiol Plant. 2009 Oct;137(2):166-74. doi: 10.1111/j.1399-3054.2009.01269.x. Epub 2009 Jul 14. Physiol Plant. 2009. PMID: 19678897
-
The fourth transmembrane domain of the Helicobacter pylori Na+/H+ antiporter NhaA faces a water-filled channel required for ion transport.J Biol Chem. 2004 Sep 24;279(39):40567-75. doi: 10.1074/jbc.M401132200. Epub 2004 Jul 19. J Biol Chem. 2004. PMID: 15263004
-
Structure and function of yeast and fungal Na+ /H+ antiporters.IUBMB Life. 2018 Jan;70(1):23-31. doi: 10.1002/iub.1701. Epub 2017 Dec 8. IUBMB Life. 2018. PMID: 29219228 Review.
-
Functional role of polar amino acid residues in Na+/H+ exchangers.Biochem J. 2001 Jul 1;357(Pt 1):1-10. doi: 10.1042/0264-6021:3570001. Biochem J. 2001. PMID: 11415429 Free PMC article. Review.
Cited by
-
Halophytes as new model plant species for salt tolerance strategies.Front Plant Sci. 2023 May 11;14:1137211. doi: 10.3389/fpls.2023.1137211. eCollection 2023. Front Plant Sci. 2023. PMID: 37251767 Free PMC article. Review.
-
Correction: Functional Analysis of the Na+,K+/H+ Antiporter PeNHX3 from the Tree Halophyte Populus euphratica in Yeast by Model-Guided Mutagenesis.PLoS One. 2015 Feb 3;10(2):e0117869. doi: 10.1371/journal.pone.0117869. eCollection 2015. PLoS One. 2015. PMID: 25646763 Free PMC article.
-
Salt-Responsive Transcriptome Profiling of Suaeda glauca via RNA Sequencing.PLoS One. 2016 Mar 1;11(3):e0150504. doi: 10.1371/journal.pone.0150504. eCollection 2016. PLoS One. 2016. PMID: 26930632 Free PMC article.
-
Genome-wide identification and drought stress-induced expression analysis of the NHX gene family in potato.Front Genet. 2024 Jul 11;15:1396375. doi: 10.3389/fgene.2024.1396375. eCollection 2024. Front Genet. 2024. PMID: 39055260 Free PMC article.
-
Structure-Guided Identification of Critical Residues in the Vacuolar Cation/Proton Antiporter NHX1 from Arabidopsis thaliana.Plants (Basel). 2023 Jul 26;12(15):2778. doi: 10.3390/plants12152778. Plants (Basel). 2023. PMID: 37570932 Free PMC article.
References
-
- Blumwald E (2000) Sodium transport and salt tolerance in plants. Curr Opin Cell Biol 12(4): 431–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

