Adenosine A2A receptors activation facilitates neuromuscular transmission in the pre-symptomatic phase of the SOD1(G93A) ALS mice, but not in the symptomatic phase
- PMID: 25093813
- PMCID: PMC4122437
- DOI: 10.1371/journal.pone.0104081
Adenosine A2A receptors activation facilitates neuromuscular transmission in the pre-symptomatic phase of the SOD1(G93A) ALS mice, but not in the symptomatic phase
Abstract
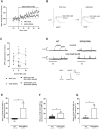
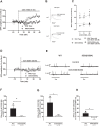
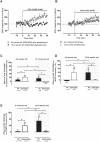
Amyotrophic Lateral Sclerosis (ALS) is a neurodegenerative disease leading to motor neuron dysfunction resulting in impairment of neuromuscular transmission. A2A adenosine receptors have already been considered as a potential therapeutical target for ALS but their neuromodulatory role at the neuromuscular junction in ALS remains to be clarified. In the present work, we evaluated the effects of A2A receptors on neuromuscular transmission of an animal model of ALS: SOD1(G93A) mice either in the pre-symptomatic (4-6 weeks old) or in the symptomatic (12-14 weeks old) stage. Electrophysiological experiments were performed obtaining intracellular recordings in Mg2+ paralyzed phrenic nerve-hemidiaphragm preparations. Endplate potentials (EPPs), quantal content (q. c.) of EPPs, miniature endplate potentials (MEPPs) and giant miniature endplate potential (GMEPPs) were recorded. In the pre-symptomatic phase of the disease (4-6 weeks old mice), the selective A2A receptor agonist, CGS 21680, significantly enhanced (p<0.05 Unpaired t-test) the mean amplitude and q.c. of EPPs, and the frequency of MEPPs and GMEPPs at SOD1(G93A) neuromuscular junctions, the effect being of higher magnitude (p<0.05, Unpaired t-test) than age-matched control littermates. On the contrary, in symptomatic mice (12-14 weeks old), CGS 21680 was devoid of effect on both the amplitude and q.c. of EPPs and the frequency of MEPPs and GMEPPs (p<0.05 Paired t-test). The results herein reported clearly document that at the neuromuscular junction of SOD1(G93A) mice there is an exacerbation of A2A receptor-mediated excitatory effects at the pre-symptomatic phase, whereas in the symptomatic phase A2A receptor activation is absent. The results thus suggest that A2A receptors function changes with ALS progression.
Conflict of interest statement
Figures
Similar articles
-
Presymptomatic and symptomatic ALS SOD1(G93A) mice differ in adenosine A1 and A2A receptor-mediated tonic modulation of neuromuscular transmission.Purinergic Signal. 2015 Dec;11(4):471-80. doi: 10.1007/s11302-015-9465-4. Epub 2015 Sep 3. Purinergic Signal. 2015. PMID: 26335190 Free PMC article.
-
Early changes of neuromuscular transmission in the SOD1(G93A) mice model of ALS start long before motor symptoms onset.PLoS One. 2013 Sep 5;8(9):e73846. doi: 10.1371/journal.pone.0073846. eCollection 2013. PLoS One. 2013. PMID: 24040091 Free PMC article.
-
Hippocampal synaptic dysfunction in the SOD1G93A mouse model of Amyotrophic Lateral Sclerosis: Reversal by adenosine A2AR blockade.Neuropharmacology. 2020 Jul;171:108106. doi: 10.1016/j.neuropharm.2020.108106. Epub 2020 Apr 18. Neuropharmacology. 2020. PMID: 32311420
-
Regulation of Acetylcholine Quantal Release by Coupled Thrombin/BDNF Signaling in Mouse Motor Synapses.Cells. 2019 Jul 22;8(7):762. doi: 10.3390/cells8070762. Cells. 2019. PMID: 31336670 Free PMC article.
-
VAChT overexpression increases acetylcholine at the synaptic cleft and accelerates aging of neuromuscular junctions.Skelet Muscle. 2016 Oct 5;6:31. doi: 10.1186/s13395-016-0105-7. eCollection 2016. Skelet Muscle. 2016. PMID: 27713817 Free PMC article.
Cited by
-
Effects of adenosine receptor overexpression and silencing in neurons and glial cells on lifespan, fitness, and sleep of Drosophila melanogaster.Exp Brain Res. 2023 Jul;241(7):1887-1904. doi: 10.1007/s00221-023-06649-y. Epub 2023 Jun 19. Exp Brain Res. 2023. PMID: 37335362 Free PMC article.
-
Amyotrophic Lateral Sclerosis (ALS) and Adenosine Receptors.Front Pharmacol. 2018 Apr 16;9:267. doi: 10.3389/fphar.2018.00267. eCollection 2018. Front Pharmacol. 2018. PMID: 29713276 Free PMC article. Review.
-
Opposite Synaptic Alterations at the Neuromuscular Junction in an ALS Mouse Model: When Motor Units Matter.J Neurosci. 2017 Sep 13;37(37):8901-8918. doi: 10.1523/JNEUROSCI.3090-16.2017. Epub 2017 Aug 11. J Neurosci. 2017. PMID: 28821658 Free PMC article.
-
How Are Adenosine and Adenosine A2A Receptors Involved in the Pathophysiology of Amyotrophic Lateral Sclerosis?Biomedicines. 2021 Aug 17;9(8):1027. doi: 10.3390/biomedicines9081027. Biomedicines. 2021. PMID: 34440231 Free PMC article. Review.
-
Swim Training Ameliorates Hyperlocomotion of ALS Mice and Increases Glutathione Peroxidase Activity in the Spinal Cord.Int J Mol Sci. 2021 Oct 27;22(21):11614. doi: 10.3390/ijms222111614. Int J Mol Sci. 2021. PMID: 34769048 Free PMC article.
References
-
- Robberecht W, Philips T (2013) The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci 14: 248–64. - PubMed
-
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, et al. (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 364: 362. - PubMed
-
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, et al. (1994) Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264: 1772–5. - PubMed
-
- Kim YI, Joo C, Cheng CC, Davis CE, O’Shaughnessy TJ (1997) Neuromuscular transmission in a transgenic animal model of motor neuron disease. Proceedings of the 18th Annual International Conference of the Ieee Engineering in Medicine and Biology Society, Vol 18, Pts 1–5 18: 1773–1774.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

