Adult mouse subventricular zones stimulate glioblastoma stem cells specific invasion through CXCL12/CXCR4 signaling
- PMID: 25085362
- PMCID: PMC4483049
- DOI: 10.1093/neuonc/nou144
Adult mouse subventricular zones stimulate glioblastoma stem cells specific invasion through CXCL12/CXCR4 signaling
Abstract
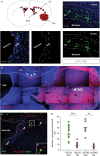
Background: Patients with glioblastoma multiforme (GBM) have an overall median survival of 15 months. This catastrophic survival rate is the consequence of systematic relapses that could arise from remaining glioblastoma stem cells (GSCs) left behind after surgery. We previously demonstrated that GSCs are able to escape the tumor mass and specifically colonize the adult subventricular zones (SVZs) after transplantation. This specific localization, away from the initial injection site, therefore represents a high-quality model of a clinical obstacle to therapy and relapses because GSCs notably retain the ability to form secondary tumors.
Method: In this work, we questioned the role of the CXCL12/CXCR4 signaling in the GSC-specific invasion of the SVZs.
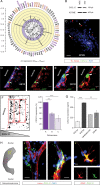
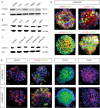
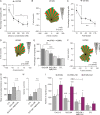
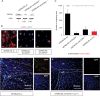
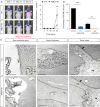
Results: We demonstrated that both receptor and ligand are respectively expressed by different GBM cell populations and by the SVZ itself. In vitro migration bio-assays highlighted that human U87MG GSCs isolated from the SVZs (U87MG-SVZ) display stronger migratory abilities in response to recombinant CXCL12 and/or SVZ-conditioned medium (SVZ-CM) compared with cancer cells isolated from the tumor mass (U87MG-TM). Moreover, in vitro inhibition of the CXCR4 signaling significantly decreased the U87MG-SVZ cell migration in response to the SVZ-CM. Very interestingly, treating U87MG-xenografted mice with daily doses of AMD3100, a specific CXCR4 antagonist, prevented the specific invasion of the SVZ. Another in vivo experiment, using CXCR4-invalidated GBM cells, displayed similar results.
Conclusion: Taken together, these data demonstrate the significant role of the CXCL12/CXCR4 signaling in this original model of brain cancer invasion.
Keywords: CXCL12; cancer stem cells; invasion; stem cell microenvironment.
© The Author(s) 2014. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
CXCL12 mediates glioblastoma resistance to radiotherapy in the subventricular zone.Neuro Oncol. 2017 Jan;19(1):66-77. doi: 10.1093/neuonc/now136. Epub 2016 Jul 1. Neuro Oncol. 2017. PMID: 27370398 Free PMC article.
-
Inhibition of CXCL12/CXCR4 autocrine/paracrine loop reduces viability of human glioblastoma stem-like cells affecting self-renewal activity.Toxicology. 2013 Dec 15;314(2-3):209-20. doi: 10.1016/j.tox.2013.10.003. Epub 2013 Oct 21. Toxicology. 2013. PMID: 24157575
-
ABCF1/CXCL12/CXCR4 Enhances Glioblastoma Cell Proliferation, Migration, and Invasion by Activating the PI3K/AKT Signal Pathway.Dev Neurosci. 2024;46(3):210-220. doi: 10.1159/000533130. Epub 2023 Sep 27. Dev Neurosci. 2024. PMID: 37757768
-
CXCL12/CXCR4 signaling in malignant brain tumors: a potential pharmacological therapeutic target.Brain Tumor Pathol. 2011 Apr;28(2):89-97. doi: 10.1007/s10014-010-0013-1. Epub 2011 Jan 6. Brain Tumor Pathol. 2011. PMID: 21210239 Review.
-
Overlapping migratory mechanisms between neural progenitor cells and brain tumor stem cells.Cell Mol Life Sci. 2019 Sep;76(18):3553-3570. doi: 10.1007/s00018-019-03149-7. Epub 2019 May 17. Cell Mol Life Sci. 2019. PMID: 31101934 Free PMC article. Review.
Cited by
-
CXCL12 mediates glioblastoma resistance to radiotherapy in the subventricular zone.Neuro Oncol. 2017 Jan;19(1):66-77. doi: 10.1093/neuonc/now136. Epub 2016 Jul 1. Neuro Oncol. 2017. PMID: 27370398 Free PMC article.
-
The splicing FK506-binding protein-51 isoform plays a role in glioblastoma resistance through programmed cell death ligand-1 expression regulation.Cell Death Discov. 2019 Sep 24;5:137. doi: 10.1038/s41420-019-0216-0. eCollection 2019. Cell Death Discov. 2019. PMID: 31583120 Free PMC article.
-
The Synergistic Effect of Combination Progesterone and Temozolomide on Human Glioblastoma Cells.PLoS One. 2015 Jun 25;10(6):e0131441. doi: 10.1371/journal.pone.0131441. eCollection 2015. PLoS One. 2015. PMID: 26110872 Free PMC article.
-
Survival and Proliferation of Neural Progenitor-Derived Glioblastomas Under Hypoxic Stress is Controlled by a CXCL12/CXCR4 Autocrine-Positive Feedback Mechanism.Clin Cancer Res. 2017 Mar 1;23(5):1250-1262. doi: 10.1158/1078-0432.CCR-15-2888. Epub 2016 Aug 19. Clin Cancer Res. 2017. PMID: 27542769 Free PMC article.
-
Opinion: Bridging gaps and doubts in glioblastoma cell-of-origin.Front Oncol. 2022 Oct 21;12:1002933. doi: 10.3389/fonc.2022.1002933. eCollection 2022. Front Oncol. 2022. PMID: 36338762 Free PMC article. No abstract available.
References
-
- Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. - PubMed
-
- Kroonen J, Nassen J, Boulanger YG, et al. Human glioblastoma-initiating cells invade specifically the subventricular zones and olfactory bulbs of mice after striatal injection. Int J Cancer. 2011;129(3):574–585. - PubMed
-
- Sadahiro H, Yoshikawa K, Ideguchi M, et al. Pathological features of highly invasive glioma stem cells in a mouse xenograft model. Brain Tumor Pathol. 2013;30(2):1–8. - PubMed
-
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–1079. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

