Central role of Snail1 in the regulation of EMT and resistance in cancer: a target for therapeutic intervention
- PMID: 25084828
- PMCID: PMC4237825
- DOI: 10.1186/s13046-014-0062-0
Central role of Snail1 in the regulation of EMT and resistance in cancer: a target for therapeutic intervention
Abstract
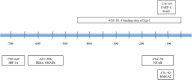
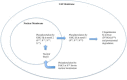
Snail1 is the founding member of the Snail superfamily of zinc-finger transcription factors, which also includes Snail2 (Slug) and Snail3 (Smuc). The superfamily is involved in cell differentiation and survival, two processes central in cancer research. Encoded by the SNAI1 gene located on human chromosome 20q13.2, Snail1 is composed of 264 amino acids and usually acts as a transcriptional repressor. Phosphorylation and nuclear localization of Snail1, governed by PI3K and Wnt signaling pathways crosstalk, are critical in Snail1's regulation. Snail1 has a pivotal role in the regulation of epithelial-mesenchymal transition (EMT), the process by which epithelial cells acquire a migratory, mesenchymal phenotype, as a result of its repression of E-cadherin. Snail1-induced EMT involves the loss of E-cadherin and claudins with concomitant upregulation of vimentin and fibronectin, among other biomarkers. While essential to normal developmental processes such as gastrulation, EMT is associated with metastasis, the cancer stem cell phenotype, and the regulation of chemo and immune resistance in cancer. Snail1 expression is a common sign of poor prognosis in metastatic cancer, and tumors with elevated Snail1 expression are disproportionately difficult to eradicate by current therapeutic treatments. The significance of Snail1 as a prognostic indicator, its involvement in the regulation of EMT and metastasis, and its roles in both drug and immune resistance point out that Snail1 is an attractive target for tumor growth inhibition and a target for sensitization to cytotoxic drugs.
Figures


Similar articles
-
Differential role of Snail1 and Snail2 zinc fingers in E-cadherin repression and epithelial to mesenchymal transition.J Biol Chem. 2014 Jan 10;289(2):930-41. doi: 10.1074/jbc.M113.528026. Epub 2013 Dec 1. J Biol Chem. 2014. PMID: 24297167 Free PMC article.
-
HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition.J Biol Chem. 2008 Nov 28;283(48):33437-46. doi: 10.1074/jbc.M802016200. Epub 2008 Oct 1. J Biol Chem. 2008. PMID: 18832382 Free PMC article.
-
Snail1 induces epithelial-to-mesenchymal transition and tumor initiating stem cell characteristics.BMC Cancer. 2011 Sep 19;11:396. doi: 10.1186/1471-2407-11-396. BMC Cancer. 2011. PMID: 21929801 Free PMC article.
-
Dual role of Snail1 as transcriptional repressor and activator.Biochim Biophys Acta Rev Cancer. 2024 Jan;1879(1):189037. doi: 10.1016/j.bbcan.2023.189037. Epub 2023 Dec 2. Biochim Biophys Acta Rev Cancer. 2024. PMID: 38043804 Review.
-
The novel role of Yin Yang 1 in the regulation of epithelial to mesenchymal transition in cancer via the dysregulated NF-κB/Snail/YY1/RKIP/PTEN Circuitry.Crit Rev Oncog. 2011;16(3-4):211-26. doi: 10.1615/critrevoncog.v16.i3-4.50. Crit Rev Oncog. 2011. PMID: 22248055 Review.
Cited by
-
Epithelial-mesenchymal plasticity (EMP) in wound healing: Exploring EMT mechanisms, regulatory network, and therapeutic opportunities.Heliyon. 2024 Jul 8;10(14):e34269. doi: 10.1016/j.heliyon.2024.e34269. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39108889 Free PMC article. Review.
-
Snail levels control the migration mechanism of mesenchymal tumor cells.Oncol Lett. 2016 Jul;12(1):767-771. doi: 10.3892/ol.2016.4642. Epub 2016 May 30. Oncol Lett. 2016. PMID: 27347214 Free PMC article.
-
Role of ARK5 in cancer and other diseases (Review).Exp Ther Med. 2021 Jul;22(1):697. doi: 10.3892/etm.2021.10129. Epub 2021 May 2. Exp Ther Med. 2021. PMID: 33986861 Free PMC article. Review.
-
A New Renieramycin T Right-Half Analog as a Small Molecule Degrader of STAT3.Mar Drugs. 2024 Aug 14;22(8):370. doi: 10.3390/md22080370. Mar Drugs. 2024. PMID: 39195486 Free PMC article.
-
HHIP's Dynamic Role in Epithelial Wound Healing Reveals a Potential Mechanism of COPD Susceptibility.bioRxiv [Preprint]. 2024 Oct 8:2024.09.05.611545. doi: 10.1101/2024.09.05.611545. bioRxiv. 2024. PMID: 39416045 Free PMC article. Preprint.
References
-
- Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. - PubMed
-
- Boulay J, Dennefeld C, Alberga A. The Drosophila developmental gene snail encodes a protein with nucleic acid binding fingers. Nature. 1987;330:395–398. - PubMed
-
- Manzanares M, Locascio A, Nieto MA. The increasing complexity of the snail gene superfamily in metazoan evolution. Trends Genet. 2001;17:178–181. - PubMed
-
- Nusslein-Volhard C, Weischaus E, Kluding H. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. I. Zygotic loci on the second chromosome. Wilheim Roux’s Arch Dev Biol. 1984;193:267–282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

