Escherichia coli-host macrophage interactions in the pathogenesis of inflammatory bowel disease
- PMID: 25083050
- PMCID: PMC4112894
- DOI: 10.3748/wjg.v20.i27.8751
Escherichia coli-host macrophage interactions in the pathogenesis of inflammatory bowel disease
Abstract
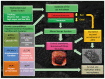
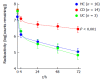
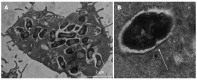
Multiple studies have demonstrated alterations in the intestinal microbial community (termed the microbiome) in Crohn's disease (CD) and several lines of evidence suggest these changes may have a significant role in disease pathogenesis. In active and quiescent disease, both the faecal and mucosa-associated microbiome are discordant with matched controls with reduced biodiversity, changes in dominant organisms and increased temporal variation described. Mucosa-associated adherent, invasive Escherichia coli (E. coli) (AIEC), pro-inflammatory and resistant to killing by mucosal macrophages, appear to be particularly important. AIEC possess several virulence factors which may confer pathogenic potential in CD. Type-1 pili (FimH) allow adherence to intestinal cells via cell-surface carcinoembryonic antigen-related cell adhesion molecules and possession of long polar fimbrae promotes translocation across the intestinal mucosa via microfold (M)-cells of the follicle-associated epithelium. Resistance to stress genes (htrA, dsbA and hfq) and tolerance of an acidic pH may contribute to survival within the phagolysosomal environment. Here we review the current understanding of the role of mucosa-associated E. coli in Crohn's pathogenesis, the role of the innate immune system, factors which may contribute to prolonged bacterial survival and therapeutic strategies to target intracellular E. coli.
Keywords: Autophagy; Crohn’s disease; Escherichia coli; Inflammatory bowel disease; Intra-macrophage survival and replication; Phagolysosome.
Figures

Similar articles
-
Point mutations in FimH adhesin of Crohn's disease-associated adherent-invasive Escherichia coli enhance intestinal inflammatory response.PLoS Pathog. 2013 Jan;9(1):e1003141. doi: 10.1371/journal.ppat.1003141. Epub 2013 Jan 24. PLoS Pathog. 2013. PMID: 23358328 Free PMC article.
-
GipA Factor Supports Colonization of Peyer's Patches by Crohn's Disease-associated Escherichia Coli.Inflamm Bowel Dis. 2016 Jan;22(1):68-81. doi: 10.1097/MIB.0000000000000609. Inflamm Bowel Dis. 2016. PMID: 26512715
-
Development of Heptylmannoside-Based Glycoconjugate Antiadhesive Compounds against Adherent-Invasive Escherichia coli Bacteria Associated with Crohn's Disease.mBio. 2015 Nov 17;6(6):e01298-15. doi: 10.1128/mBio.01298-15. mBio. 2015. PMID: 26578673 Free PMC article.
-
Adherent-Invasive E. coli: Update on the Lifestyle of a Troublemaker in Crohn's Disease.Int J Mol Sci. 2020 May 25;21(10):3734. doi: 10.3390/ijms21103734. Int J Mol Sci. 2020. PMID: 32466328 Free PMC article. Review.
-
Understanding host-adherent-invasive Escherichia coli interaction in Crohn's disease: opening up new therapeutic strategies.Biomed Res Int. 2014;2014:567929. doi: 10.1155/2014/567929. Epub 2014 Dec 15. Biomed Res Int. 2014. PMID: 25580435 Free PMC article. Review.
Cited by
-
Oral immune therapy: targeting the systemic immune system via the gut immune system for the treatment of inflammatory bowel disease.Clin Transl Immunology. 2016 Jan 29;5(1):e60. doi: 10.1038/cti.2015.47. eCollection 2016 Jan. Clin Transl Immunology. 2016. PMID: 26900473 Free PMC article. Review.
-
FKBP11 protects intestinal epithelial cells against inflammation‑induced apoptosis via the JNK‑caspase pathway in Crohn's disease.Mol Med Rep. 2018 Nov;18(5):4428-4438. doi: 10.3892/mmr.2018.9485. Epub 2018 Sep 14. Mol Med Rep. 2018. PMID: 30221722 Free PMC article.
-
Interplay between Serotonin, Immune Response, and Intestinal Dysbiosis in Inflammatory Bowel Disease.Int J Mol Sci. 2022 Dec 9;23(24):15632. doi: 10.3390/ijms232415632. Int J Mol Sci. 2022. PMID: 36555276 Free PMC article. Review.
-
An adult zebrafish model for adherent-invasive Escherichia coli indicates protection from AIEC infection by probiotic E. coli Nissle.iScience. 2022 Jun 9;25(7):104572. doi: 10.1016/j.isci.2022.104572. eCollection 2022 Jul 15. iScience. 2022. PMID: 35769878 Free PMC article.
-
The Crohn's disease-related bacterial strain LF82 assembles biofilm-like communities to protect itself from phagolysosomal attack.Commun Biol. 2021 May 25;4(1):627. doi: 10.1038/s42003-021-02161-7. Commun Biol. 2021. PMID: 34035436 Free PMC article.
References
-
- Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–1605. - PubMed
-
- Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. - PubMed
-
- Canavan C, Abrams KR, Mayberry JF. Meta-analysis: mortality in Crohn’s disease. Aliment Pharmacol Ther. 2007;25:861–870. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

