Mitochondria-mediated apoptosis in mammals
- PMID: 25073422
- PMCID: PMC4180462
- DOI: 10.1007/s13238-014-0089-1
Mitochondria-mediated apoptosis in mammals
Abstract
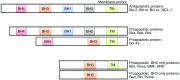
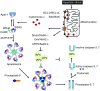
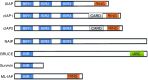
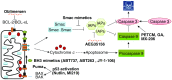
The mitochondria-mediated caspase activation pathway is a major apoptotic pathway characterized by mitochondrial outer membrane permeabilization (MOMP) and subsequent release of cytochrome c into the cytoplasm to activate caspases. MOMP is regulated by the Bcl-2 family of proteins. This pathway plays important roles not only in normal development, maintenance of tissue homeostasis and the regulation of immune system, but also in human diseases such as immune disorders, neurodegeneration and cancer. In the past decades the molecular basis of this pathway and the regulatory mechanism have been comprehensively studied, yet a great deal of new evidence indicates that cytochrome c release from mitochondria does not always lead to irreversible cell death, and that caspase activation can also have non-death functions. Thus, many unsolved questions and new challenges are still remaining. Furthermore, the dysfunction of this pathway involved in cancer development is obvious, and targeting the pathway as a therapeutic strategy has been extensively explored, but the efficacy of the targeted therapies is still under development. In this review we will discuss the mitochondria-mediated apoptosis pathway and its physiological roles and therapeutic implications.
Figures
Similar articles
-
Mitochondria-dependent apoptosis induced by nanoscale hydroxyapatite in human gastric cancer SGC-7901 cells.Biol Pharm Bull. 2007 Jan;30(1):128-32. doi: 10.1248/bpb.30.128. Biol Pharm Bull. 2007. PMID: 17202672
-
Bax/Bak-dependent, Drp1-independent Targeting of X-linked Inhibitor of Apoptosis Protein (XIAP) into Inner Mitochondrial Compartments Counteracts Smac/DIABLO-dependent Effector Caspase Activation.J Biol Chem. 2015 Sep 4;290(36):22005-18. doi: 10.1074/jbc.M115.643064. Epub 2015 Jul 1. J Biol Chem. 2015. PMID: 26134559 Free PMC article.
-
Goniothalamin induces cell cycle arrest and apoptosis in H400 human oral squamous cell carcinoma: A caspase-dependent mitochondrial-mediated pathway with downregulation of NF-κβ.Arch Oral Biol. 2016 Apr;64:28-38. doi: 10.1016/j.archoralbio.2015.12.002. Epub 2015 Dec 23. Arch Oral Biol. 2016. PMID: 26752226
-
Clearing the final hurdles to mitochondrial apoptosis: regulation post cytochrome C release.Exp Oncol. 2012 Oct;34(3):185-91. Exp Oncol. 2012. PMID: 23070003 Review.
-
Cytochrome C-mediated apoptosis.Annu Rev Biochem. 2004;73:87-106. doi: 10.1146/annurev.biochem.73.011303.073706. Annu Rev Biochem. 2004. PMID: 15189137 Review.
Cited by
-
Mitochondrial Dynamics Imbalance: A Strategy for Promoting Viral Infection.Front Microbiol. 2020 Aug 21;11:1992. doi: 10.3389/fmicb.2020.01992. eCollection 2020. Front Microbiol. 2020. PMID: 32973718 Free PMC article. Review.
-
A glimpse into viral warfare: decoding the intriguing role of highly pathogenic coronavirus proteins in apoptosis regulation.J Biomed Sci. 2024 Jul 13;31(1):70. doi: 10.1186/s12929-024-01062-1. J Biomed Sci. 2024. PMID: 39003473 Free PMC article. Review.
-
A potential role of X-linked inhibitor of apoptosis protein in mitochondrial membrane permeabilization and its implication in cancer therapy.Drug Discov Today. 2016 Jan;21(1):38-47. doi: 10.1016/j.drudis.2015.07.014. Epub 2015 Jul 30. Drug Discov Today. 2016. PMID: 26232549 Free PMC article. Review.
-
Exercise and Metformin Intervention Prevents Lipotoxicity-Induced Hepatocyte Apoptosis by Alleviating Oxidative and ER Stress and Activating the AMPK/Nrf2/HO-1 Signaling Pathway in db/db Mice.Oxid Med Cell Longev. 2022 Sep 9;2022:2297268. doi: 10.1155/2022/2297268. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36120597 Free PMC article.
-
Valproic acid induces neuronal cell death through a novel calpain-dependent necroptosis pathway.J Neurochem. 2015 Apr;133(2):174-86. doi: 10.1111/jnc.13029. Epub 2015 Feb 8. J Neurochem. 2015. PMID: 25581256 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

