Host and viral RNA-binding proteins involved in membrane targeting, replication and intercellular movement of plant RNA virus genomes
- PMID: 25071804
- PMCID: PMC4083346
- DOI: 10.3389/fpls.2014.00321
Host and viral RNA-binding proteins involved in membrane targeting, replication and intercellular movement of plant RNA virus genomes
Abstract
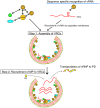
Many plant viruses have positive-strand RNA [(+)RNA] as their genome. Therefore, it is not surprising that RNA-binding proteins (RBPs) play important roles during (+)RNA virus infection in host plants. Increasing evidence demonstrates that viral and host RBPs play critical roles in multiple steps of the viral life cycle, including translation and replication of viral genomic RNAs, and their intra- and intercellular movement. Although studies focusing on the RNA-binding activities of viral and host proteins, and their associations with membrane targeting, and intercellular movement of viral genomes have been limited to a few viruses, these studies have provided important insights into the molecular mechanisms underlying the replication and movement of viral genomic RNAs. In this review, we briefly overview the currently defined roles of viral and host RBPs whose RNA-binding activity have been confirmed experimentally in association with their membrane targeting, and intercellular movement of plant RNA virus genomes.
Keywords: RNA replication; RNA virus; RNA-binding protein; cell-to-cell movement; cellular membrane.
Figures
Similar articles
-
Diverse roles of host RNA binding proteins in RNA virus replication.RNA Biol. 2011 Mar-Apr;8(2):305-15. doi: 10.4161/rna.8.2.15391. Epub 2011 Mar 1. RNA Biol. 2011. PMID: 21505273 Free PMC article. Review.
-
Pathogenesis mediated by proviral host factors involved in translation and replication of plant positive-strand RNA viruses.Curr Opin Virol. 2016 Apr;17:11-18. doi: 10.1016/j.coviro.2015.11.004. Epub 2015 Nov 30. Curr Opin Virol. 2016. PMID: 26651023 Review.
-
Small hydrophobic viral proteins involved in intercellular movement of diverse plant virus genomes.AIMS Microbiol. 2020 Sep 21;6(3):305-329. doi: 10.3934/microbiol.2020019. eCollection 2020. AIMS Microbiol. 2020. PMID: 33134746 Free PMC article. Review.
-
Intercellular movement of plant RNA viruses: Targeting replication complexes to the plasmodesma for both accuracy and efficiency.Traffic. 2020 Dec;21(12):725-736. doi: 10.1111/tra.12768. Epub 2020 Oct 22. Traffic. 2020. PMID: 33090653 Review.
-
Plant virus replication and movement.Virology. 2015 May;479-480:657-71. doi: 10.1016/j.virol.2015.01.025. Epub 2015 Mar 3. Virology. 2015. PMID: 25746797 Review.
Cited by
-
Localization and subcellular association of Grapevine Pinot Gris Virus in grapevine leaf tissues.Protoplasma. 2018 May;255(3):923-935. doi: 10.1007/s00709-017-1198-5. Epub 2017 Dec 22. Protoplasma. 2018. PMID: 29273825 Free PMC article.
-
The Intriguing Conundrum of a Nonconserved Multifunctional Protein of Citrus Tristeza Virus That Interacts with a Viral Long Non-Coding RNA.Viruses. 2021 Oct 22;13(11):2129. doi: 10.3390/v13112129. Viruses. 2021. PMID: 34834936 Free PMC article.
-
Host Factors Involved in the Intracellular Movement of Bamboo mosaic virus.Front Microbiol. 2017 Apr 25;8:759. doi: 10.3389/fmicb.2017.00759. eCollection 2017. Front Microbiol. 2017. PMID: 28487692 Free PMC article. Review.
-
Harnessing host ROS-generating machinery for the robust genome replication of a plant RNA virus.Proc Natl Acad Sci U S A. 2017 Feb 14;114(7):E1282-E1290. doi: 10.1073/pnas.1610212114. Epub 2017 Feb 1. Proc Natl Acad Sci U S A. 2017. PMID: 28154139 Free PMC article.
-
Insights Into Natural Genetic Resistance to Rice Yellow Mottle Virus and Implications on Breeding for Durable Resistance.Front Plant Sci. 2021 Jun 29;12:671355. doi: 10.3389/fpls.2021.671355. eCollection 2021. Front Plant Sci. 2021. PMID: 34267770 Free PMC article. Review.
References
-
- An M., Iwakawa H.-O., Mine A., Kaido M., Mise K., Okuno T. (2010). A Y-shaped RNA structure in the 3’ untranslated region together with the trans-activator and core promoter of Red clover necrotic mosaic virus RNA2 is required for its negative-strand RNA synthesis. Virology 405 100–109 10.1016/j.virol.2010.05.022 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

