Synthesis and folding of a mirror-image enzyme reveals ambidextrous chaperone activity
- PMID: 25071217
- PMCID: PMC4136631
- DOI: 10.1073/pnas.1410900111
Synthesis and folding of a mirror-image enzyme reveals ambidextrous chaperone activity
Abstract
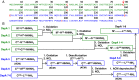
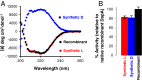
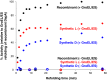
Mirror-image proteins (composed of D-amino acids) are promising therapeutic agents and drug discovery tools, but as synthesis of larger D-proteins becomes feasible, a major anticipated challenge is the folding of these proteins into their active conformations. In vivo, many large and/or complex proteins require chaperones like GroEL/ES to prevent misfolding and produce functional protein. The ability of chaperones to fold D-proteins is unknown. Here we examine the ability of GroEL/ES to fold a synthetic d-protein. We report the total chemical synthesis of a 312-residue GroEL/ES-dependent protein, DapA, in both L- and D-chiralities, the longest fully synthetic proteins yet reported. Impressively, GroEL/ES folds both L- and D-DapA. This work extends the limits of chemical protein synthesis, reveals ambidextrous GroEL/ES folding activity, and provides a valuable tool to fold d-proteins for drug development and mirror-image synthetic biology applications.
Keywords: peptide synthesis; protein folding.
Conflict of interest statement
Conflict of interest statement: M.S.K. is a Scientific Director, consultant, and equity holder of the
Figures
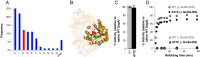

Similar articles
-
GroEL/ES chaperonin modulates the mechanism and accelerates the rate of TIM-barrel domain folding.Cell. 2014 May 8;157(4):922-934. doi: 10.1016/j.cell.2014.03.038. Cell. 2014. PMID: 24813614 Free PMC article.
-
Molecular chaperone GroEL/ES: unfolding and refolding processes.Biochemistry (Mosc). 2013 Dec;78(13):1405-14. doi: 10.1134/S0006297913130038. Biochemistry (Mosc). 2013. PMID: 24490731 Review.
-
Overproduction of the Escherichia coli Chaperones GroEL-GroES in Rhodococcus ruber Improves the Activity and Stability of Cell Catalysts Harboring a Nitrile Hydratase.J Microbiol Biotechnol. 2016 Feb;26(2):337-46. doi: 10.4014/jmb.1509.09084. J Microbiol Biotechnol. 2016. PMID: 26562693
-
Unfolded DapA forms aggregates when diluted into free solution, confounding comparison with folding by the GroEL/GroES chaperonin system.FEBS Lett. 2015 Feb 13;589(4):497-499. doi: 10.1016/j.febslet.2015.01.008. Epub 2015 Jan 17. FEBS Lett. 2015. PMID: 25601566 Free PMC article.
-
Interferon-gamma is a target for binding and folding by both Escherichia coli chaperone model systems GroEL/GroES and DnaK/DnaJ/GrpE.Biochimie. 1998 Aug-Sep;80(8-9):729-37. doi: 10.1016/s0300-9084(99)80026-1. Biochimie. 1998. PMID: 9865495 Review.
Cited by
-
Protein chemical synthesis by α-ketoacid-hydroxylamine ligation.Nat Protoc. 2016 Jun;11(6):1130-47. doi: 10.1038/nprot.2016.052. Epub 2016 May 26. Nat Protoc. 2016. PMID: 27227514
-
Enhancing native chemical ligation for challenging chemical protein syntheses.Curr Opin Chem Biol. 2020 Oct;58:37-44. doi: 10.1016/j.cbpa.2020.04.003. Epub 2020 Jul 31. Curr Opin Chem Biol. 2020. PMID: 32745915 Free PMC article. Review.
-
Aligator: A computational tool for optimizing total chemical synthesis of large proteins.Bioorg Med Chem. 2017 Sep 15;25(18):4946-4952. doi: 10.1016/j.bmc.2017.05.061. Epub 2017 Jun 3. Bioorg Med Chem. 2017. PMID: 28651912 Free PMC article. Review.
-
Channel activity of mirror-image M2 proton channel of influenza A virus is blocked by achiral or chiral inhibitors.Protein Cell. 2019 Mar;10(3):211-216. doi: 10.1007/s13238-018-0536-5. Protein Cell. 2019. PMID: 29679235 Free PMC article. No abstract available.
-
Mirror-image polymerase chain reaction.Cell Discov. 2017 Oct 17;3:17037. doi: 10.1038/celldisc.2017.37. eCollection 2017. Cell Discov. 2017. PMID: 29051832 Free PMC article.
References
-
- Zawadzke LE, Berg JM. A racemic protein. J Am Chem Soc. 1992;114(10):4002–4003.
-
- Milton RC, Milton SC, Kent SB. Total chemical synthesis of a D-enzyme: The enantiomers of HIV-1 protease show reciprocal chiral substrate specificity. Science. 1992;256(5062):1445–1448. - PubMed
-
- Schumacher TN, et al. Identification of D-peptide ligands through mirror-image phage display. Science. 1996;271(5257):1854–1857. - PubMed
-
- Verzele D, Madder A. Patchwork protein chemistry: A practitioner’s treatise on the advances in synthetic peptide stitchery. ChemBioChem. 2013;14(9):1032–1048. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

