Slik and the receptor tyrosine kinase Breathless mediate localized activation of Moesin in terminal tracheal cells
- PMID: 25061859
- PMCID: PMC4111555
- DOI: 10.1371/journal.pone.0103323
Slik and the receptor tyrosine kinase Breathless mediate localized activation of Moesin in terminal tracheal cells
Abstract
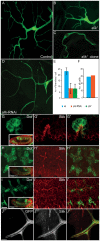
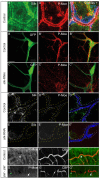
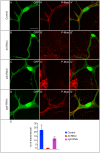
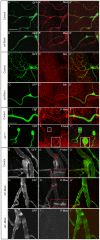
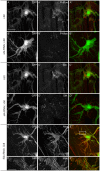
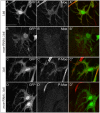
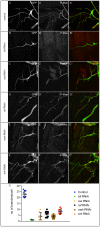
A key element in the regulation of subcellular branching and tube morphogenesis of the Drosophila tracheal system is the organization of the actin cytoskeleton by the ERM protein Moesin. Activation of Moesin within specific subdomains of cells, critical for its interaction with actin, is a tightly controlled process and involves regulatory inputs from membrane proteins, kinases and phosphatases. The kinases that activate Moesin in tracheal cells are not known. Here we show that the Sterile-20 like kinase Slik, enriched at the luminal membrane, is necessary for the activation of Moesin at the luminal membrane and regulates branching and subcellular tube morphogenesis of terminal cells. Our results reveal the FGF-receptor Breathless as an additional necessary cue for the activation of Moesin in terminal cells. Breathless-mediated activation of Moesin is independent of the canonical MAP kinase pathway.
Conflict of interest statement
Figures
Similar articles
-
Fascin links Btl/FGFR signalling to the actin cytoskeleton during Drosophila tracheal morphogenesis.Development. 2014 Feb;141(4):929-39. doi: 10.1242/dev.103218. Development. 2014. PMID: 24496629
-
Drosophila dopamine synthesis pathway genes regulate tracheal morphogenesis.Dev Biol. 2007 Aug 1;308(1):30-43. doi: 10.1016/j.ydbio.2007.04.047. Epub 2007 May 3. Dev Biol. 2007. PMID: 17585895 Free PMC article.
-
STRIPAK regulates Slik localization to control mitotic morphogenesis and epithelial integrity.J Cell Biol. 2020 Nov 2;219(11):e201911035. doi: 10.1083/jcb.201911035. J Cell Biol. 2020. PMID: 32960945 Free PMC article.
-
Branching morphogenesis in the Drosophila tracheal system.Cold Spring Harb Symp Quant Biol. 1997;62:241-7. Cold Spring Harb Symp Quant Biol. 1997. PMID: 9598357 Review. No abstract available.
-
Drosophila gastrulation: identification of a missing link.Curr Biol. 2004 Jun 22;14(12):R480-2. doi: 10.1016/j.cub.2004.06.016. Curr Biol. 2004. PMID: 15203022 Review.
Cited by
-
Development and Function of the Drosophila Tracheal System.Genetics. 2018 Jun;209(2):367-380. doi: 10.1534/genetics.117.300167. Genetics. 2018. PMID: 29844090 Free PMC article. Review.
-
Crosstalk between basal extracellular matrix adhesion and building of apical architecture during morphogenesis.Biol Open. 2021 Nov 15;10(11):bio058760. doi: 10.1242/bio.058760. Epub 2021 Nov 29. Biol Open. 2021. PMID: 34842274 Free PMC article. Review.
-
Intracellular lumen formation in Drosophila proceeds via a novel subcellular compartment.Development. 2015 Nov 15;142(22):3964-73. doi: 10.1242/dev.127902. Epub 2015 Oct 1. Development. 2015. PMID: 26428009 Free PMC article.
-
C-terminal phosphorylation modulates ERM-1 localization and dynamics to control cortical actin organization and support lumen formation during Caenorhabditiselegans development.Development. 2020 Jul 22;147(14):dev188011. doi: 10.1242/dev.188011. Development. 2020. PMID: 32586975 Free PMC article.
-
Slik maintains tissue homeostasis by preventing JNK-mediated apoptosis.Cell Div. 2023 Oct 4;18(1):16. doi: 10.1186/s13008-023-00097-4. Cell Div. 2023. PMID: 37794497 Free PMC article.
References
-
- Tsukita S, Yonemura S (1999) Cortical actin organization: lessons from ERM (ezrin/radixin/moesin) proteins. J Biol Chem 274: 34507–34510. - PubMed
-
- Louvet-Vallee S (2000) ERM proteins: from cellular architecture to cell signaling. Biol Cell 92: 305–316. - PubMed
-
- Bretscher A, Edwards K, Fehon RG (2002) ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3: 586–599. - PubMed
-
- Polesello C, Payre F (2004) Small is beautiful: what flies tell us about ERM protein function in development. Trends Cell Biol 14: 294–302. - PubMed
-
- Fehon R (2006) Cell biology: polarity bites. Nature 442: 519–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

