Antibody to gp41 MPER alters functional properties of HIV-1 Env without complete neutralization
- PMID: 25058619
- PMCID: PMC4110039
- DOI: 10.1371/journal.ppat.1004271
Antibody to gp41 MPER alters functional properties of HIV-1 Env without complete neutralization
Abstract
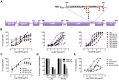
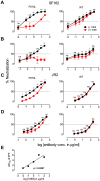
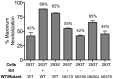
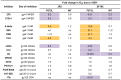
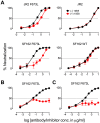
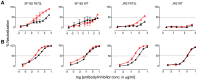
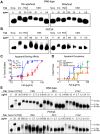
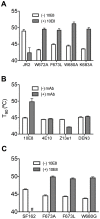
Human antibody 10E8 targets the conserved membrane proximal external region (MPER) of envelope glycoprotein (Env) subunit gp41 and neutralizes HIV-1 with exceptional potency. Remarkably, HIV-1 containing mutations that reportedly knockout 10E8 binding to linear MPER peptides are partially neutralized by 10E8, producing a local plateau in the dose response curve. Here, we found that virus partially neutralized by 10E8 becomes significantly less neutralization sensitive to various MPER antibodies and to soluble CD4 while becoming significantly more sensitive to antibodies and fusion inhibitors against the heptad repeats of gp41. Thus, 10E8 modulates sensitivity of Env to ligands both pre- and post-receptor engagement without complete neutralization. Partial neutralization by 10E8 was influenced at least in part by perturbing Env glycosylation. With unliganded Env, 10E8 bound with lower apparent affinity and lower subunit occupancy to MPER mutant compared to wild type trimers. However, 10E8 decreased functional stability of wild type Env while it had an opposite, stabilizing effect on MPER mutant Envs. Clade C isolates with natural MPER polymorphisms also showed partial neutralization by 10E8 with altered sensitivity to various gp41-targeted ligands. Our findings suggest a novel mechanism of virus neutralization by demonstrating how antibody binding to the base of a trimeric spike cross talks with adjacent subunits to modulate Env structure and function. The ability of an antibody to stabilize, destabilize, partially neutralize as well as alter neutralization sensitivity of a virion spike pre- and post-receptor engagement may have implications for immunotherapy and vaccine design.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Functional Optimization of Broadly Neutralizing HIV-1 Antibody 10E8 by Promotion of Membrane Interactions.J Virol. 2018 Mar 28;92(8):e02249-17. doi: 10.1128/JVI.02249-17. Print 2018 Apr 15. J Virol. 2018. PMID: 29386285 Free PMC article.
-
Mechanism of HIV-1 neutralization by antibodies targeting a membrane-proximal region of gp41.J Virol. 2014 Jan;88(2):1249-58. doi: 10.1128/JVI.02664-13. Epub 2013 Nov 13. J Virol. 2014. PMID: 24227838 Free PMC article.
-
Lipid interactions and angle of approach to the HIV-1 viral membrane of broadly neutralizing antibody 10E8: Insights for vaccine and therapeutic design.PLoS Pathog. 2017 Feb 22;13(2):e1006212. doi: 10.1371/journal.ppat.1006212. eCollection 2017 Feb. PLoS Pathog. 2017. PMID: 28225819 Free PMC article.
-
Neutralizing Antibodies Targeting HIV-1 gp41.Viruses. 2020 Oct 23;12(11):1210. doi: 10.3390/v12111210. Viruses. 2020. PMID: 33114242 Free PMC article. Review.
-
Antigp41 membrane proximal external region antibodies and the art of using the membrane for neutralization.Curr Opin HIV AIDS. 2017 May;12(3):250-256. doi: 10.1097/COH.0000000000000364. Curr Opin HIV AIDS. 2017. PMID: 28422789 Review.
Cited by
-
Induction of Heterologous Tier 2 HIV-1-Neutralizing and Cross-Reactive V1/V2-Specific Antibodies in Rabbits by Prime-Boost Immunization.J Virol. 2016 Sep 12;90(19):8644-60. doi: 10.1128/JVI.00853-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27440894 Free PMC article.
-
HIV-1 Envelope and MPER Antibody Structures in Lipid Assemblies.Cell Rep. 2020 Apr 28;31(4):107583. doi: 10.1016/j.celrep.2020.107583. Cell Rep. 2020. PMID: 32348769 Free PMC article.
-
To bnAb or Not to bnAb: Defining Broadly Neutralising Antibodies Against HIV-1.Front Immunol. 2021 Oct 19;12:708227. doi: 10.3389/fimmu.2021.708227. eCollection 2021. Front Immunol. 2021. PMID: 34737737 Free PMC article. Review.
-
Immunogenic Display of Purified Chemically Cross-Linked HIV-1 Spikes.J Virol. 2015 Jul;89(13):6725-45. doi: 10.1128/JVI.03738-14. Epub 2015 Apr 15. J Virol. 2015. PMID: 25878116 Free PMC article.
-
DNA prime/protein boost vaccination elicits robust humoral response in rhesus macaques using oligomeric simian immunodeficiency virus envelope and Advax delta inulin adjuvant.J Gen Virol. 2017 Aug;98(8):2143-2155. doi: 10.1099/jgv.0.000863. Epub 2017 Jul 31. J Gen Virol. 2017. PMID: 28758637 Free PMC article.
References
-
- Layne SP, Merges MJ, Dembo M, Spouge JL, Conley SR, et al. (1992) Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology 189: 695–714. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

