Inhibitory action of benzo[α]pyrene on hepatic lipoprotein receptors in vitro and on liver lipid homeostasis in mice
- PMID: 25054229
- PMCID: PMC4108373
- DOI: 10.1371/journal.pone.0102991
Inhibitory action of benzo[α]pyrene on hepatic lipoprotein receptors in vitro and on liver lipid homeostasis in mice
Abstract
Background: Dyslipidemia associated with obesity often manifests as increased plasma LDL and triglyceride-rich lipoprotein levels suggesting changes in hepatic lipoprotein receptor status. Persistent organic pollutants have been recently postulated to contribute to the obesity etiology by increasing adipogenesis, but little information is available on their potential effect on hepatic lipoprotein metabolism.
Objective: The objective of this study was to investigate the effect of the common environmental pollutant, benzo[α]pyrene (B[α]P) on two lipoprotein receptors, the LDL-receptor and the lipolysis-stimulated lipoprotein receptor (LSR) as well as the ATP-binding cassette transporter A1 (ABCA1) using cell and animal models.
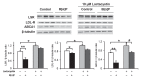
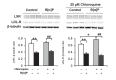
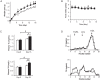
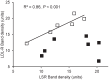
Results: LSR, LDL-receptor as well as ABCA1 protein levels were significantly decreased by 26-48% in Hepa1-6 cells incubated (<2 h) in the presence of B[α]P (≤1 µM). Real-time PCR analysis and lactacystin studies revealed that this effect was due primarily to increased proteasome, and not lysosomal-mediated degradation rather than decreased transcription. Furthermore, ligand blots revealed that lipoproteins exposed to 1 or 5 µM B[α]P displayed markedly decreased (42-86%) binding to LSR or LDL-receptor. B[α]P-treated (0.5 mg/kg/48 h, i.p. 15 days) C57BL/6J mice displayed higher weight gain, associated with significant increases in plasma cholesterol, triglycerides, and liver cholesterol content, and decreased hepatic LDL-receptor and ABCA1 levels. Furthermore, correlational analysis revealed that B[α]P abolished the positive association observed in control mice between the LSR and LDL-receptor. Interestingly, levels of other proteins involved in liver cholesterol metabolism, ATP-binding cassette transporter G1 and scavenger receptor-BI, were decreased, while those of acyl-CoA:cholesterol acyltransferase 1 and 2 were increased in B[α]P-treated mice.
Conclusions: B[α]P demonstrates inhibitory action on LSR and LDL-R, as well as ABCA1, which we propose leads to modified lipid status in B[α]P-treated mice, thus providing new insight into mechanisms underlying the involvement of pollutants in the disruption of lipid homeostasis, potentially contributing to dyslipidemia associated with obesity.
Conflict of interest statement
Figures


Similar articles
-
Lipolysis stimulated lipoprotein receptor: a novel molecular link between hyperlipidemia, weight gain, and atherosclerosis in mice.J Biol Chem. 2008 Sep 12;283(37):25650-25659. doi: 10.1074/jbc.M801027200. Epub 2008 Jul 21. J Biol Chem. 2008. PMID: 18644789
-
Aortic cholesterol accumulation correlates with systemic inflammation but not hepatic and gonadal adipose tissue inflammation in low-density lipoprotein receptor null mice.Nutr Res. 2013 Dec;33(12):1072-82. doi: 10.1016/j.nutres.2013.09.002. Nutr Res. 2013. PMID: 24267047 Free PMC article.
-
The high-fat high-fructose hamster as an animal model for niacin's biological activities in humans.Metabolism. 2013 Dec;62(12):1840-9. doi: 10.1016/j.metabol.2013.08.001. Epub 2013 Sep 13. Metabolism. 2013. PMID: 24035454
-
Up-regulation of hepatic lipolysis stimulated lipoprotein receptor by leptin: a potential lever for controlling lipid clearance during the postprandial phase.FASEB J. 2010 Nov;24(11):4218-28. doi: 10.1096/fj.10-160440. Epub 2010 Jul 20. FASEB J. 2010. PMID: 20647547
-
Foam cells in atherosclerosis.Clin Chim Acta. 2013 Sep 23;424:245-52. doi: 10.1016/j.cca.2013.06.006. Epub 2013 Jun 16. Clin Chim Acta. 2013. PMID: 23782937 Review.
Cited by
-
Garcinia cambogia Extract Increased Hepatic Levels of Lipolysis-Stimulated Lipoprotein Receptor and Lipids in Mice on Normal Diet.Int J Mol Sci. 2023 Nov 14;24(22):16298. doi: 10.3390/ijms242216298. Int J Mol Sci. 2023. PMID: 38003494 Free PMC article.
-
Acceleration of benzo(a)pyrene-induced colon carcinogenesis by Western diet in a rat model of colon cancer.Curr Res Toxicol. 2024 Mar 7;6:100162. doi: 10.1016/j.crtox.2024.100162. eCollection 2024. Curr Res Toxicol. 2024. PMID: 38496007 Free PMC article.
-
Western diet enhances benzo(a)pyrene-induced colon tumorigenesis in a polyposis in rat coli (PIRC) rat model of colon cancer.Oncotarget. 2016 May 17;7(20):28947-60. doi: 10.18632/oncotarget.7901. Oncotarget. 2016. PMID: 26959117 Free PMC article.
References
-
- Caballero B (2007) The global epidemic of obesity: an overview. Epidemiol Rev 29: 1–5. - PubMed
-
- Cunningham E (2010) Where can I find obesity statistics? J Am Diet Assoc 110: 656. - PubMed
-
- Howard BV, Ruotolo G, Robbins DC (2003) Obesity and dyslipidemia. Endocrinol Metab Clin North Am 32: 855–867. - PubMed
-
- Eckel RH (2011) The complex metabolic mechanisms relating obesity to hypertriglyceridemia. Arterioscler Thromb Vasc Biol 31: 1946–1948. - PubMed
-
- Brown MS, Goldstein JL (1986) A receptor-mediated pathway for cholesterol homeostasis. Science 232: 34–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

