Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in breast cancer: possibilities for therapeutic intervention
- PMID: 25051360
- PMCID: PMC4148087
- DOI: 10.18632/oncotarget.2209
Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in breast cancer: possibilities for therapeutic intervention
Abstract
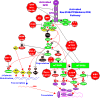
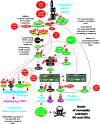
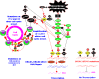
The EGFR/PI3K/PTEN/Akt/mTORC1/GSK-3 pathway plays prominent roles in malignant transformation, prevention of apoptosis, drug resistance and metastasis. The expression of this pathway is frequently altered in breast cancer due to mutations at or aberrant expression of: HER2, ERalpha, BRCA1, BRCA2, EGFR1, PIK3CA, PTEN, TP53, RB as well as other oncogenes and tumor suppressor genes. In some breast cancer cases, mutations at certain components of this pathway (e.g., PIK3CA) are associated with a better prognosis than breast cancers lacking these mutations. The expression of this pathway and upstream HER2 has been associated with breast cancer initiating cells (CICs) and in some cases resistance to treatment. The anti-diabetes drug metformin can suppress the growth of breast CICs and herceptin-resistant HER2+ cells. This review will discuss the importance of the EGFR/PI3K/PTEN/Akt/mTORC1/GSK-3 pathway primarily in breast cancer but will also include relevant examples from other cancer types. The targeting of this pathway will be discussed as well as clinical trials with novel small molecule inhibitors. The targeting of the hormone receptor, HER2 and EGFR1 in breast cancer will be reviewed in association with suppression of the EGFR/PI3K/PTEN/Akt/mTORC1/GSK-3 pathway.
Figures
Similar articles
-
Ran is a potential therapeutic target for cancer cells with molecular changes associated with activation of the PI3K/Akt/mTORC1 and Ras/MEK/ERK pathways.Clin Cancer Res. 2012 Jan 15;18(2):380-91. doi: 10.1158/1078-0432.CCR-11-2035. Epub 2011 Nov 16. Clin Cancer Res. 2012. PMID: 22090358 Free PMC article.
-
Frequent PTEN genomic alterations and activated phosphatidylinositol 3-kinase pathway in basal-like breast cancer cells.Breast Cancer Res. 2008;10(6):R101. doi: 10.1186/bcr2204. Epub 2008 Dec 3. Breast Cancer Res. 2008. PMID: 19055754 Free PMC article.
-
PIK3CA mutations, phosphatase and tensin homolog, human epidermal growth factor receptor 2, and insulin-like growth factor 1 receptor and adjuvant tamoxifen resistance in postmenopausal breast cancer patients.Breast Cancer Res. 2014 Jan 27;16(1):R13. doi: 10.1186/bcr3606. Breast Cancer Res. 2014. PMID: 24467828 Free PMC article. Clinical Trial.
-
Targeting the translational apparatus to improve leukemia therapy: roles of the PI3K/PTEN/Akt/mTOR pathway.Leukemia. 2011 Jul;25(7):1064-79. doi: 10.1038/leu.2011.46. Epub 2011 Mar 25. Leukemia. 2011. PMID: 21436840 Review.
-
Mosaic Disorders of the PI3K/PTEN/AKT/TSC/mTORC1 Signaling Pathway.Dermatol Clin. 2017 Jan;35(1):51-60. doi: 10.1016/j.det.2016.07.001. Dermatol Clin. 2017. PMID: 27890237 Free PMC article. Review.
Cited by
-
JARID1B promotes metastasis and epithelial-mesenchymal transition via PTEN/AKT signaling in hepatocellular carcinoma cells.Oncotarget. 2015 May 20;6(14):12723-39. doi: 10.18632/oncotarget.3713. Oncotarget. 2015. PMID: 25909289 Free PMC article.
-
Tumor suppression in basal keratinocytes via dual non-cell-autonomous functions of a Na,K-ATPase beta subunit.Elife. 2016 May 30;5:e14277. doi: 10.7554/eLife.14277. Elife. 2016. PMID: 27240166 Free PMC article.
-
Synthesis, characterization and bioactivity of new pyridine-2(H)-one, nicotinonitrile, and furo[2,3-b]pyridine derivatives.Mol Divers. 2024 Jul 16. doi: 10.1007/s11030-024-10934-5. Online ahead of print. Mol Divers. 2024. PMID: 39009909
-
Genistein as Potential Therapeutic Candidate for Menopausal Symptoms and Other Related Diseases.Molecules. 2019 Oct 29;24(21):3892. doi: 10.3390/molecules24213892. Molecules. 2019. PMID: 31671813 Free PMC article. Review.
-
ANLN and UBE2T are prognostic biomarkers associated with immune regulation in breast cancer: a bioinformatics analysis.Cancer Cell Int. 2022 May 16;22(1):193. doi: 10.1186/s12935-022-02611-0. Cancer Cell Int. 2022. PMID: 35578283 Free PMC article.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Weiss JR, Moysich KB, Swede H. Epidemiology of male breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:20–26. - PubMed
-
- Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, Bell R, Rosenthal J, Hussey C, Tran T, McClure M, Fry C, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

