The centrosome-Golgi apparatus nexus
- PMID: 25047616
- PMCID: PMC4113106
- DOI: 10.1098/rstb.2013.0462
The centrosome-Golgi apparatus nexus
Abstract
A shared feature among all microtubule (MT)-dependent processes is the requirement for MTs to be organized in arrays of defined geometry. At a fundamental level, this is achieved by precisely controlling the timing and localization of the nucleation events that give rise to new MTs. To this end, MT nucleation is restricted to specific subcellular sites called MT-organizing centres. The primary MT-organizing centre in proliferating animal cells is the centrosome. However, the discovery of MT nucleation capacity of the Golgi apparatus (GA) has substantially changed our understanding of MT network organization in interphase cells. Interestingly, MT nucleation at the Golgi apparently relies on multiprotein complexes, similar to those present at the centrosome, that assemble at the cis-face of the organelle. In this process, AKAP450 plays a central role, acting as a scaffold to recruit other centrosomal proteins important for MT generation. MT arrays derived from either the centrosome or the GA differ in their geometry, probably reflecting their different, yet complementary, functions. Here, I review our current understanding of the molecular mechanisms involved in MT nucleation at the GA and how Golgi- and centrosome-based MT arrays work in concert to ensure the formation of a pericentrosomal polarized continuous Golgi ribbon structure, a critical feature for cell polarity in mammalian cells. In addition, I comment on the important role of the Golgi-nucleated MTs in organizing specialized MT arrays that serve specific functions in terminally differentiated cells.
Keywords: AKAP450; Golgi apparatus; centrosome; microtubules.
Figures
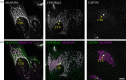
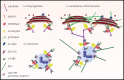
Similar articles
-
Microtubule nucleation at the cis-side of the Golgi apparatus requires AKAP450 and GM130.EMBO J. 2009 Apr 22;28(8):1016-28. doi: 10.1038/emboj.2009.47. Epub 2009 Feb 26. EMBO J. 2009. PMID: 19242490 Free PMC article.
-
Disconnecting the Golgi ribbon from the centrosome prevents directional cell migration and ciliogenesis.J Cell Biol. 2011 May 30;193(5):917-33. doi: 10.1083/jcb.201011014. Epub 2011 May 23. J Cell Biol. 2011. PMID: 21606206 Free PMC article.
-
The dual role of the centrosome in organizing the microtubule network in interphase.EMBO Rep. 2018 Nov;19(11):e45942. doi: 10.15252/embr.201845942. Epub 2018 Sep 17. EMBO Rep. 2018. PMID: 30224411 Free PMC article.
-
Regulatory mechanisms and cellular functions of non-centrosomal microtubules.J Biochem. 2017 Jul 1;162(1):1-10. doi: 10.1093/jb/mvx018. J Biochem. 2017. PMID: 28338985 Review.
-
Golgi as an MTOC: making microtubules for its own good.Histochem Cell Biol. 2013 Sep;140(3):361-7. doi: 10.1007/s00418-013-1119-4. Epub 2013 Jul 3. Histochem Cell Biol. 2013. PMID: 23821162 Free PMC article. Review.
Cited by
-
The factory, the antenna and the scaffold: the three-way interplay between the Golgi, cilium and extracellular matrix underlying tissue function.Biol Open. 2023 Feb 15;12(2):bio059719. doi: 10.1242/bio.059719. Epub 2023 Feb 21. Biol Open. 2023. PMID: 36802341 Free PMC article. Review.
-
Misplaced Golgi Elements Produce Randomly Oriented Microtubules and Aberrant Cortical Arrays of Microtubules in Dystrophic Skeletal Muscle Fibers.Front Cell Dev Biol. 2019 Sep 18;7:176. doi: 10.3389/fcell.2019.00176. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31620435 Free PMC article.
-
Actin- and microtubule-dependent regulation of Golgi morphology by FHDC1.Mol Biol Cell. 2016 Jan 15;27(2):260-76. doi: 10.1091/mbc.E15-02-0070. Epub 2015 Nov 12. Mol Biol Cell. 2016. PMID: 26564798 Free PMC article.
-
The centrosomal linker and microtubules provide dual levels of spatial coordination of centrosomes.PLoS Genet. 2015 May 22;11(5):e1005243. doi: 10.1371/journal.pgen.1005243. eCollection 2015 May. PLoS Genet. 2015. PMID: 26001056 Free PMC article.
-
Targeting Drosophila Sas6 to mitochondria reveals its high affinity for Gorab.Biol Open. 2022 Nov 1;11(11):bio059545. doi: 10.1242/bio.059545. Epub 2022 Nov 18. Biol Open. 2022. PMID: 36331102 Free PMC article.
References
-
- Moudjou M, Bordes N, Paintrand M, Bornens M. 1996. gamma-Tubulin in mammalian cells: the centrosomal and the cytosolic forms. J. Cell Sci. 109, 875–887. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

