Conservative therapy with metformin plus megestrol acetate for endometrial atypical hyperplasia
- PMID: 25045434
- PMCID: PMC4102740
- DOI: 10.3802/jgo.2014.25.3.214
Conservative therapy with metformin plus megestrol acetate for endometrial atypical hyperplasia
Abstract
Objective: To compare the efficacy of metformin plus megestrol acetate (MA) with that of MA alone for treating endometrial atypical hyperplasia (EAH).
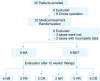
Methods: This pilot study included 16 EAH patients who met at least one metabolic syndrome (MS) criterion and received either adjunctive metformin plus MA (MET group) or MA monotherapy (MA group). Each patient in the MA group received 160 mg of MA daily, whereas patients in the MET group received the same dose of MA plus 0.5 g of metformin thrice daily. Treatment response was assessed by histological examination of dilation and curettage specimens obtained after 12 weeks of therapy.
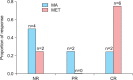
Results: Each group had eight patients, and half of the patients in each group were diagnosed with MS. The complete response (CR) rate was 75% (6/8) in the MET group and 25% (2/8) in the MA group (p=0.105). Complications of MS did not affect the response rates in either group. In the MET group, 75% (3/4) of the patients had CR in the presence or absence of MS. In the MA group, 50% (2/4) of the patients with MS had CR, whereas no patient without MS had CR. No irreversible toxicities were observed.
Conclusion: Metformin plus MA may be a potential alternative therapy for treating EAH, and the MS status of patients may have no effect on the efficacy of metformin plus MA therapy.
Keywords: Endometrial hyperplasia; Megestrol acetate; Metformin.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

Comment in
-
Conservative treatment for atypical endometrial hyperplasia: what is the most effective therapeutic method?J Gynecol Oncol. 2014 Jul;25(3):164-5. doi: 10.3802/jgo.2014.25.3.164. J Gynecol Oncol. 2014. PMID: 25045426 Free PMC article. No abstract available.
Similar articles
-
Metformin plus megestrol acetate compared with megestrol acetate alone as fertility-sparing treatment in patients with atypical endometrial hyperplasia and well-differentiated endometrial cancer: a randomised controlled trial.BJOG. 2020 Jun;127(7):848-857. doi: 10.1111/1471-0528.16108. Epub 2020 Feb 16. BJOG. 2020. PMID: 31961463 Clinical Trial.
-
[The long-term efficacy of metformin in megestrol acetate-based fertility-sparing treatment for patients with endometrial atypical hyperplasia and endometrioid endometrial cancer].Zhonghua Yi Xue Za Zhi. 2024 Mar 12;104(10):729-735. doi: 10.3760/cma.j.cn112137-20231016-00768. Zhonghua Yi Xue Za Zhi. 2024. PMID: 38462352 Clinical Trial. Chinese.
-
The impact of adjunctive metformin to progesterone for the treatment of non-atypical endometrial hyperplasia in a randomized fashion, a placebo-controlled, double blind clinical trial.J Gynecol Obstet Hum Reprod. 2021 Jun;50(6):101863. doi: 10.1016/j.jogoh.2020.101863. Epub 2020 Jul 8. J Gynecol Obstet Hum Reprod. 2021. PMID: 32652300 Clinical Trial.
-
Conservative management of grade 2 stage IA endometrial carcinoma and literature review.J Obstet Gynaecol Res. 2021 Mar;47(3):984-991. doi: 10.1111/jog.14646. Epub 2021 Jan 5. J Obstet Gynaecol Res. 2021. PMID: 33403812 Review.
-
Effects of metformin on endometrial cancer: Systematic review and meta-analysis.Gynecol Oncol. 2017 Oct;147(1):167-180. doi: 10.1016/j.ygyno.2017.07.120. Epub 2017 Jul 29. Gynecol Oncol. 2017. PMID: 28760367 Review.
Cited by
-
Metformin for endometrial hyperplasia.Cochrane Database Syst Rev. 2024 May 2;5(5):CD012214. doi: 10.1002/14651858.CD012214.pub3. Cochrane Database Syst Rev. 2024. PMID: 38695827 Review.
-
Comparison among fertility-sparing therapies for well differentiated early-stage endometrial carcinoma and complex atypical hyperplasia.Oncotarget. 2017 May 3;8(34):57642-57653. doi: 10.18632/oncotarget.17588. eCollection 2017 Aug 22. Oncotarget. 2017. PMID: 28915701 Free PMC article. Review.
-
Metformin for endometrial hyperplasia.Cochrane Database Syst Rev. 2017 Oct 27;10(10):CD012214. doi: 10.1002/14651858.CD012214.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD012214. doi: 10.1002/14651858.CD012214.pub3 PMID: 29077194 Free PMC article. Updated. Review.
-
Levonorgestrel-releasing intrauterine device therapy vs oral progestin treatment for reproductive-aged patients with endometrial intraepithelial neoplasia: a systematic review and meta-analysis.J Natl Cancer Inst. 2024 May 8;116(5):653-664. doi: 10.1093/jnci/djae023. J Natl Cancer Inst. 2024. PMID: 38305500 Free PMC article.
-
Fertility sparing treatment for early stage endometrial cancer: current situation and new strategy.J Gynecol Oncol. 2019 Nov;30(6):e117. doi: 10.3802/jgo.2019.30.e117. J Gynecol Oncol. 2019. PMID: 31576702 Free PMC article. No abstract available.
References
-
- Baker J, Obermair A, Gebski V, Janda M. Efficacy of oral or intrauterine device-delivered progestin in patients with complex endometrial hyperplasia with atypia or early endometrial adenocarcinoma: a meta-analysis and systematic review of the literature. Gynecol Oncol. 2012;125:263–270. - PubMed
-
- Nucera G, Mandato VD, Gelli MC, Palomba S, La Sala GB. Gonadotropin releasing hormone agonist and levonorgestrel-intrauterine device followed by in vitro fertilization program as management strategy for an infertile endometrial cancer patient: a case report. Gynecol Endocrinol. 2013;29:219–221. - PubMed
-
- Jasonni VM, Franceschetti F, Ciotti P, Bulletti C, Vignudelli A, Marabini A, et al. Treatment of endometrial hyperplasia with cyproterone acetate histological and hormonal aspects. Acta Obstet Gynecol Scand. 1986;65:685–687. - PubMed
-
- Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, Saito T, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007;25:2798–2803. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

