Inhaled corticosteroids in children with persistent asthma: effects on growth
- PMID: 25030198
- PMCID: PMC8407362
- DOI: 10.1002/14651858.CD009471.pub2
Inhaled corticosteroids in children with persistent asthma: effects on growth
Abstract
Background: Treatment guidelines for asthma recommend inhaled corticosteroids (ICS) as first-line therapy for children with persistent asthma. Although ICS treatment is generally considered safe in children, the potential systemic adverse effects related to regular use of these drugs have been and continue to be a matter of concern, especially the effects on linear growth.
Objectives: To assess the impact of ICS on the linear growth of children with persistent asthma and to explore potential effect modifiers such as characteristics of available treatments (molecule, dose, length of exposure, inhalation device) and of treated children (age, disease severity, compliance with treatment).
Search methods: We searched the Cochrane Airways Group Specialised Register of trials (CAGR), which is derived from systematic searches of bibliographic databases including CENTRAL, MEDLINE, EMBASE, CINAHL, AMED and PsycINFO; we handsearched respiratory journals and meeting abstracts. We also conducted a search of ClinicalTrials.gov and manufacturers' clinical trial databases to look for potential relevant unpublished studies. The literature search was conducted in January 2014.
Selection criteria: Parallel-group randomised controlled trials comparing daily use of ICS, delivered by any type of inhalation device for at least three months, versus placebo or non-steroidal drugs in children up to 18 years of age with persistent asthma.
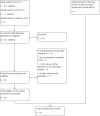
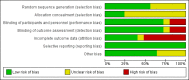
Data collection and analysis: Two review authors independently performed study selection, data extraction and assessment of risk of bias in included studies. We conducted meta-analyses using the Cochrane statistical package RevMan 5.2 and Stata version 11.0. We used the random-effects model for meta-analyses. We used mean differences (MDs) and 95% CIs as the metrics for treatment effects. A negative value for MD indicates that ICS have suppressive effects on linear growth compared with controls. We performed a priori planned subgroup analyses to explore potential effect modifiers, such as ICS molecule, daily dose, inhalation device and age of the treated child.
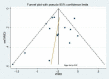
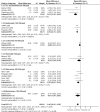
Main results: We included 25 trials involving 8471 (5128 ICS-treated and 3343 control) children with mild to moderate persistent asthma. Six molecules (beclomethasone dipropionate, budesonide, ciclesonide, flunisolide, fluticasone propionate and mometasone furoate) [corrected] given at low or medium daily doses were used during a period of three months to four to six years. Most trials were blinded and over half of the trials had drop out rates of over 20%.Compared with placebo or non-steroidal drugs, ICS produced a statistically significant reduction in linear growth velocity (14 trials with 5717 participants, MD -0.48 cm/y, 95% CI -0.65 to -0.30, moderate quality evidence) and in the change from baseline in height (15 trials with 3275 participants; MD -0.61 cm/y, 95% CI -0.83 to -0.38, moderate quality evidence) during a one-year treatment period.Subgroup analysis showed a statistically significant group difference between six molecules in the mean reduction of linear growth velocity during one-year treatment (Chi² = 26.1, degrees of freedom (df) = 5, P value < 0.0001). The group difference persisted even when analysis was restricted to the trials using doses equivalent to 200 μg/d hydrofluoroalkane (HFA)-beclomethasone. Subgroup analyses did not show a statistically significant impact of daily dose (low vs medium), inhalation device or participant age on the magnitude of ICS-induced suppression of linear growth velocity during a one-year treatment period. However, head-to-head comparisons are needed to assess the effects of different drug molecules, dose, inhalation device or patient age. No statistically significant difference in linear growth velocity was found between participants treated with ICS and controls during the second year of treatment (five trials with 3174 participants; MD -0.19 cm/y, 95% CI -0.48 to 0.11, P value 0.22). Of two trials that reported linear growth velocity in the third year of treatment, one trial involving 667 participants showed similar growth velocity between the budesonide and placebo groups (5.34 cm/y vs 5.34 cm/y), and another trial involving 1974 participants showed lower growth velocity in the budesonide group compared with the placebo group (MD -0.33 cm/y, 95% CI -0.52 to -0.14, P value 0.0005). Among four trials reporting data on linear growth after treatment cessation, three did not describe statistically significant catch-up growth in the ICS group two to four months after treatment cessation. One trial showed accelerated linear growth velocity in the fluticasone group at 12 months after treatment cessation, but there remained a statistically significant difference of 0.7 cm in height between the fluticasone and placebo groups at the end of the three-year trial.One trial with follow-up into adulthood showed that participants of prepubertal age treated with budesonide 400 μg/d for a mean duration of 4.3 years had a mean reduction of 1.20 cm (95% CI -1.90 to -0.50) in adult height compared with those treated with placebo.
Authors' conclusions: Regular use of ICS at low or medium daily doses is associated with a mean reduction of 0.48 cm/y in linear growth velocity and a 0.61-cm change from baseline in height during a one-year treatment period in children with mild to moderate persistent asthma. The effect size of ICS on linear growth velocity appears to be associated more strongly with the ICS molecule than with the device or dose (low to medium dose range). ICS-induced growth suppression seems to be maximal during the first year of therapy and less pronounced in subsequent years of treatment. However, additional studies are needed to better characterise the molecule dependency of growth suppression, particularly with newer molecules (mometasone, ciclesonide), to specify the respective role of molecule, daily dose, inhalation device and patient age on the effect size of ICS, and to define the growth suppression effect of ICS treatment over a period of several years in children with persistent asthma.
Conflict of interest statement
No conflicts of interest are known.
Figures






















Republished in
-
Inhaled corticosteroids in children with persistent asthma: effects on growth.Evid Based Child Health. 2014 Dec;9(4):829-930. doi: 10.1002/ebch.1988. Evid Based Child Health. 2014. PMID: 25504972
Comment in
-
When will research measure up to our need to know how steroids affect childhood growth?Cochrane Database Syst Rev. 2014 Jul 17;2014(7):ED000086. doi: 10.1002/14651858.ED000086. Cochrane Database Syst Rev. 2014. PMID: 25101367 Free PMC article. No abstract available.
Similar articles
-
Inhaled corticosteroids in children with persistent asthma: effects on growth.Evid Based Child Health. 2014 Dec;9(4):829-930. doi: 10.1002/ebch.1988. Evid Based Child Health. 2014. PMID: 25504972
-
Inhaled corticosteroids in children with persistent asthma: dose-response effects on growth.Cochrane Database Syst Rev. 2014 Jul 17;2014(7):CD009878. doi: 10.1002/14651858.CD009878.pub2. Cochrane Database Syst Rev. 2014. PMID: 25030199 Free PMC article. Review.
-
Inhaled corticosteroids in children with persistent asthma: effects of different drugs and delivery devices on growth.Cochrane Database Syst Rev. 2019 Jun 10;6(6):CD010126. doi: 10.1002/14651858.CD010126.pub2. Cochrane Database Syst Rev. 2019. PMID: 31194879 Free PMC article.
-
Inhaled corticosteroids in children with persistent asthma: dose-response effects on growth.Evid Based Child Health. 2014 Dec;9(4):931-1046. doi: 10.1002/ebch.1989. Evid Based Child Health. 2014. PMID: 25504973
-
Ciclesonide versus other inhaled corticosteroids for chronic asthma in children.Cochrane Database Syst Rev. 2013 Feb 28;2013(2):CD010352. doi: 10.1002/14651858.CD010352. Cochrane Database Syst Rev. 2013. PMID: 23450613 Free PMC article. Review.
Cited by
-
Inhaled corticosteroids in children with persistent asthma: Effects on growth.Paediatr Child Health. 2015 Jun-Jul;20(5):248-50. doi: 10.1093/pch/20.5.248. Paediatr Child Health. 2015. PMID: 26175562 Free PMC article. No abstract available.
-
Growth failure and treatment in cystic fibrosis.J Cyst Fibros. 2019 Oct;18 Suppl 2(Suppl 2):S82-S87. doi: 10.1016/j.jcf.2019.08.010. J Cyst Fibros. 2019. PMID: 31679733 Free PMC article. Review.
-
Neuropsychiatric adverse drug reactions in children initiated on montelukast in real-life practice.Eur Respir J. 2017 Aug 17;50(2):1700148. doi: 10.1183/13993003.00148-2017. Print 2017 Aug. Eur Respir J. 2017. PMID: 28818882
-
Different oral corticosteroid regimens for acute asthma.Cochrane Database Syst Rev. 2016 May 13;2016(5):CD011801. doi: 10.1002/14651858.CD011801.pub2. Cochrane Database Syst Rev. 2016. PMID: 27176676 Free PMC article. Review.
-
Stepping down inhaled corticosteroids from scheduled to as needed in stable asthma: Systematic review and meta-analysis.Allergy Asthma Proc. 2015 Jul-Aug;36(4):262-7. doi: 10.2500/aap.2015.36.3850. Allergy Asthma Proc. 2015. PMID: 26108083 Free PMC article. Review.
References
References to studies included in this review
Allen 1998 {published data only}
-
- Allen DB, Bronsky EA, LaForce CF, Nathan RA, Tinkelman DG, Vandewalker ML, et al. Growth in asthmatic children treated with fluticasone propionate. Fluticasone Propionate Asthma Study Group. The Journal of Pediatrics 1998;132(3 Pt 1):472‐7. - PubMed
Becker 2006 {published data only}
-
- Becker AB, Kuznetsova O, Vermeulen J, Soto‐Quiros ME, Young B, Reiss TF, et al. Linear growth in prepubertal asthmatic children treated with montelukast, beclomethasone, or placebo: a 56‐week randomized double‐blind study. Annals of Allergy, Asthma & Immunology 2006;96(6):800‐7. - PubMed
Bensch 2011 {published data only}
-
- Bensch GW, Greos LS, Gawchik S, Kpamegan E, Newman KB. Linear growth and bone maturation are unaffected by 1 year of therapy with inhaled flunisolide hydrofluoroalkane in prepubescent children with mild persistent asthma: a randomized, double‐blind, placebo‐controlled trial. Annals of Allergy, Asthma & Immunology 2011;107(4):323‐9. - PubMed
Bisgaard 2004 {published data only}
-
- Bisgaard H, Allen D, Milanowski J, Kalev I, Willits L, Davies P. Twelve‐month safety and efficacy of Inhaled fluticasone propionate in children aged 1 to 3 years with recurrent wheezing. Pediatrics 2004;113:e87. - PubMed
CAMP 2000 {published data only}
-
- The childhood asthma management program research group. Long‐term effects of budesonide or nedocromil in children with asthma. The New England Journal of Medicine 2000;343(15):1054‐63. - PubMed
Doull 1995 {published data only}
-
- Doull IJ, Freezer NJ, Holgate ST. Growth of prepubertal children with mild asthma treated with inhaled beclomethasone dipropionate. American Journal of Respirtory and Critical Care Medicine 1995;151(1):1715‐9. - PubMed
Gillman 2002 {published data only}
-
- Gillman SA, Anolik R, Schenkel E, Newman K. One‐year trial on safety and normal linear growth with flunisolide HFA in children with asthma. Clinical Pediatrics 2002;41(5):333‐40. - PubMed
Gradman 2010 {published data only}
-
- Gradman J, Wolthers OD. A randomized trial of lower leg and height growth in children with asthma treated with inhaled budesonide from a new dry powder inhaler. Pediatric Allergy and Immunology 2010;21(1 Pt 2):e206‐12. - PubMed
Guilbert 2006 {published data only}
-
- Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long‐term inhaled corticosteroids in preschool children at high risk for asthma. The New England Journal of Medicine 2006;354(19):1985‐97. - PubMed
Jonasson 2000 {published data only}
-
- Jónasson G, Carlsen KH, Jonasson C, Mowinckel P. Low‐dose inhaled budesonide once or twice daily for 27 months in children with mild asthma. Allergy 2000;55(8):740‐8. - PubMed
Kannisto 2000 {published data only}
-
- Kannisto S, Korppi M, Remes K, Voutilainen R. Adrenal suppression, evaluated by a low dose adrenocorticotropin test, and growth in asthmatic children treated with inhaled steroids. The Journal of Clinical Endocrinology and Metabolism 2000;85(2):652‐7. - PubMed
-
- Kannisto S, Voutilainen R, Remes K, Korppi M. Efficacy and safety of inhaled steroid and cromone treatment in school‐age children: a randomized pragmatic pilot study. Pediatric Allergy and Immunology 2001;13:24‐30. - PubMed
Martinez 2011 {published data only}
Pauwels 2003 {published data only}
-
- Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double‐blind trial. Lancet 2003;361(9363):1071‐6. - PubMed
Pedersen 2010 {published data only}
-
- Pedersen S, Potter P, Dachev S, Bosheva M, Kaczmarek J, Springer E, et al. Efficacy and safety of three ciclesonide doses vs placebo in children with asthma: the RAINBOW study. Respiratory Medicine 2010;104:1618‐28. - PubMed
Price 1997 {published data only}
-
- Price JF, Russell G, Hindmarsh PC, Weller P, Heaf DP, Williams J. Growth during one year of treatment with fluticasone propionate or sodium cromoglycate in children with asthma. Pediatric Pulmonology 1997;24(3):178‐86. - PubMed
Roux 2003 {published data only}
-
- Roux C, Kolta S, Desfougères JL, Minini P, Bidat E. Long‐term safety of fluticasone propionate and nedocromil sodium on bone in children with asthma. Pediatrics 2003;111(6 Pt 1):e706‐13. - PubMed
Simons 1997 {published data only}
-
- Simons FE. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. Canadian Beclomethasone Dipropionate‐Salmeterol Xinafoate Study Group. New England Journal of Medicine 1997;337(23):1659‐65. - PubMed
Skoner 2008 {published data only}
-
- Skoner DP, Maspero J, Banerji D, Ciclesonide Pediatric Growth Study Group. Assessment of the long‐term safety of inhaled ciclesonide on growth in children with asthma. Pediatrics 2008;121(1):e1‐14. - PubMed
Skoner 2011 {published data only}
-
- Skoner DP, Meltzer EO, Milgrom H, Stryszak P, Teper A, Staudinger H. Effects of inhaled mometasone furoate on growth velocity and adrenal function: a placebo‐controlled trial in children 4‐9 years old with mild persistent asthma. Journal of Asthma 2011;48(8):848‐59. - PubMed
Sorkness 2007 {published data only}
-
- Sorkness CA, Lemanske RF Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long‐term comparison of 3 controller regimens for mild‐moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. Journal of Allergy and Clinical Immunology 2007;119(1):64‐72. - PubMed
Storr 1986 {published data only}
Tinkelman 1993 {published data only}
-
- Tinkelman DG, Reed CE, Nelson HS, Offord KP. Aerosol beclomethasone dipropionate compared with theophylline as primary treatment of chronic, mild to moderately severe asthma in children. Pediatrics 1993;92(1):64‐77. - PubMed
Turpeinen 2008 {published data only}
Verberne 1997 {published data only}
-
- Verberne AA, Frost C, Roorda RJ, Laag H, Kerrebijn KF. One year treatment with salmeterol compared with beclomethasone in children with asthma. The Dutch Paediatric Asthma Study Group. American Journal of Respiratory and Critical Care Medicine 1997;156(3 Pt 1):688‐95. - PubMed
Wasserman 2006 {published data only}
-
- Wasserman RL, Baker JW, Kim KT, Blake KV, Scott CA, Wu W, et al. Efficacy and safety of inhaled fluticasone propionate chlorofluorocarbon in 2‐ to 4‐year‐old patients with asthma: results of a double‐blind, placebo‐controlled study. Annals of Allergy, Asthma & Immunology 2006;96(6):808‐18. - PubMed
References to studies excluded from this review
Agertoft 1994 {published data only}
-
- Agertoft L, Pedersen S. Effects of long‐term treatment with an inhaled corticosteroid on growth and pulmonary function in asthmatic children. Respiratory Medicine 1994;88:373‐81. - PubMed
Baxter‐Jones 2000 {published data only}
-
- Baxter‐Jones AD, Helms PJ, Russell G, Grant A, Ross S, Cairns JA, et al. Early asthma prophylaxis, natural history, skeletal development and economy (EASE): a pilot randomised controlled trial. Health Technology Assessment 2000;4(28):1‐89. - PubMed
Brand 2011 {published data only}
-
- Brand PL, Luz García‐García M, Morison A, Vermeulen JH, Weber HC. Ciclesonide in wheezy preschool children with a positive asthma predictive index or atopy. Respiratory Medicine 2011;105:1588‐95. - PubMed
CAMP 1999 {published data only}
-
- CAMP Group. The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Childhood Asthma Management Program Research Group. Controlled Clinical Trials 1999;20(1):91‐120. - PubMed
Heuck 1998 {published data only}
-
- Heuck C, Wolthers OD, Kollerup G, Hansen M, Teisner B. Adverse effects of inhaled budesonide (800 micrograms) on growth and collagen turnover in children with asthma: a double‐blind comparison of once‐daily versus twice‐daily administration. Journal of Pediatrics 1998;133(5):608‐12. - PubMed
Kerrebijn 1976 {published data only}
-
- Kerrebijn KF. Beclomethasone dipropionate in long‐term treatment of asthma in children. Journal of Pediatrics 1976;89(5):821‐6. - PubMed
Martinati 1998 {published data only}
-
- Martinati LC, Bertoldo F, Gasperi E, Fortunati P, Lo Cascio V, Boner AL. Longitudinal evaluation of bone mass in asthmatic children treated with inhaled beclomethasone dipropionate or cromolyn sodium. Allergy 1998;53:705‐8. - PubMed
Merkus 1993 {published data only}
-
- Merkus PJ, Essen‐Zandvliet EE, Duiverman EJ, Houwelingen HC, Kerrebijn KF, Quanjer PH. Long‐term effect of inhaled corticosteroids on growth rate in adolescents with asthma. Pediatrics 1993;91(6):1121‐6. - PubMed
Rao 1999 {published data only}
-
- Rao R, Gregson RK, Jones AC, Miles EA, Campbell MJ, Warner JO. Systemic effects of inhaled corticosteroids on growth and bone turnover in childhood asthma: a comparison of fluticasone with beclomethasone. European Respiratory Journal 1999;13(1):87‐94. - PubMed
Teper 2004 {published data only}
-
- Teper AM, Colom AJ, Kofman CD, Maffey AF, Vidaurreta SM, Bergadá I. Effects of inhaled fluticasone propionate in children less than 2 years old with recurrent wheezing. Pediatric Pulmonology 2004;37:111‐5. - PubMed
Tinkelman 1996 {published data only}
-
- Tinkelman D. Theophylline therapy for children with asthma. European Respiratory Review 1996;6(34):79‐83.
Verberne 1998 {published data only}
-
- Verberne AA, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. The Dutch Asthma Study Group. American Journal of Respiratory and Critical Care Medicine 1998;158(1):213‐9. - PubMed
Xu 2005 {published data only}
-
- Xu Z, Chen HY, Zhang SY, Wang XL, He CR, Qiao YX. Efficacy and safety of corticosteroid inhalation in the treatment of childhood asthma. Chinese Journal of Contemporary Pediatrics 2005;7(1):47‐50.
Additional references
Adams 2011a
Adams 2011b
Adams 2011c
-
- Adams NP, Bestall JB, Jones PW. Budesonide versus placebo for chronic asthma in children and adults. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003274] - DOI
Agertoft 2000
-
- Agertoft L, Pedersen S. Effect of long‐term treatment with inhaled budesonide on adult height in children with asthma. New England Journal of Medicine 2000;343:1064‐9. - PubMed
Allen 1994
-
- Allen DB, Mullen M, Mullen B. A meta‐analysis of the effect of oral and inhaled corticosteroids on growth. Journal of Allergy and Clinical Immunology 1994;93(6):967‐76. - PubMed
Allen 1999
-
- Allen DB. Limitations of short‐term studies in predicting long‐term adverse effects of inhaled corticosteroids. Allergy 1999;54:29‐34. - PubMed
Allen 2002
-
- Allen DB. Safety of inhaled corticosteroids in children. Pediatric Pulmonology 2002;33:208‐20. - PubMed
Allen 2006
-
- Allen DB. Effects of inhaled steroids on growth, bone metabolism, and adrenal function. Advances in Pediatrics 2006;53:101‐10. - PubMed
Asher 2010
-
- Asher MI. Recent perspectives on global epidemiology of asthma in childhood. Allergologia et Immunopathologia 2010;38(2):83‐7. - PubMed
Axelsson 2013
Barnes 2006
Braman 2006
-
- Braman SS. The global burden of asthma. Chest 2006;130:4S‐12S. - PubMed
Brand 2001
-
- Brand PLP. Inhaled corticosteroids reduce growth. Or do they?. European Respiratory Journal 2001;17:287‐94. - PubMed
BTS & SIGN 2012
-
- British Thoracic Society and Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma. http://www.brit‐thoracic.org.uk/Portals/0/Guidelines/ AsthmaGuidelines/ sign101%20Jan%202012.pdf. (accessed 26 April 2013).
Bush 2004
-
- Bush A. Phenotype specific treatment of asthma in children. Paediatric Respiratory Reviews 2004;5(Supplement 1):S93‐S101. - PubMed
Busse 2001
-
- Busse WW, Lemanske RF. Asthma. New England Journal of Medicine 2001;344(5):350‐62. - PubMed
Carlsen 2002
-
- Carlsen KH, Gerritsen J. Inhaled steroids in children: adrenal suppression and growth impairment. European Respiratory Journal 2002;19:985‐8. - PubMed
Chauhan 2013
Colice 2000
-
- Colice GL. Comparing inhaled corticosteroids. Respiratory Care 2000;45(7):846‐53. - PubMed
Covar 2003
-
- Covar RA, Szefler SJ, Martin RJ, Sundstrom DA, Silkoff PE, Murphy J, et al. Relations between exhaled nitric oxide and measures of disease activity among children with mild‐to‐moderate asthma. Journal of Pediatrics 2003;142(5):469‐75. - PubMed
Creese 2001
-
- Creese KH, Doull IJ. Effects of inhaled corticosteroids on growth in asthmatic children. Current Allergy and Asthma Reports 2001;1(2):122‐6. - PubMed
Djukanovic 1992
-
- Djukanović R, Wilson JW, Britten KM, Wilson SJ, Walls AF, Roche WR, et al. Effect of an inhaled corticosteroid on airway inflammation and symptoms in asthma. American Review of Respiratory Disease 1992;145(3):669‐74. - PubMed
Efthimiou 1998
-
- Efthimiou J, Barnes PJ. Effect of inhaled corticosteroids on bones and growth. European Respiratory Journal 1998;11:1167‐77. - PubMed
FDA 1998
-
- US Food, Drug Administration (FDA). FDA requires new pediatric labelling for inhaled, intranasal corticosteroids. FDA Talk Paper November 9, 1998.
FDA 2007
-
- FDA. Guidance for Industry. Orally inhaled and intranasal corticosteroids: evaluation of the effects on growth in children. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati... (accessed 16 December 2013).
Ferguson 1999
-
- Ferguson AC, Spier S, Manjra A, Versteegh FG, Mark S, Zhang P. Efficacy and safety of high‐dose inhaled steroids in children with asthma: a comparison of fluticasone propionate with budesonide. Journal of Pediatrics 1999;134(4):422‐7. - PubMed
GINA 2012
-
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention 2012 (update). www.ginasthma.com.org (accessed 22 April 2012).
GINA 2014
-
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention, revised 2014. www.ginasthma.com.org (accessed 01 May 2014).
Hedlin 1998
-
- Hedlin G, Ingemansson M, Brännegord M, Marcus C, Stierna P. A study of children with asthma treated with inhaled corticosteroids (ICS) with or without growth retardation. Journal of Allergy and Clinical Immunology 1998;101:S13.
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. www.cochrane‐handbook.org, 2011.
Hoekx 1996
-
- Hoekx JCM, Hedlin G, Pedersen W, Sorva R, Hollingworth K, Efthimiou J. Fluticasone propionate compared with budesonide: a double‐blind trial in asthmatic children using powder devices at a dosage of 400 μg/day. European Respiratory Journal 1996;9:2263‐72. - PubMed
Hogger 2003
-
- Hogger P. Dose response and therapeutic index of inhaled corticosteroids in asthma. Current Opinion in Pulmonary Medicine 2003;9:1‐8. - PubMed
ISAAC 1998
-
- ISAAC Steering Committee. Worldwide variations in the prevalence of asthma symptoms: the International Study of Asthma and Allergies in Childhood (ISAAC). European Respiratory Journal 1998;12:315‐35. - PubMed
Juniper 1990
-
- Juniper EF, Kline PA, Vanzieleghem MA, Ramsdale EH, O'Byrne PM, Hargreave FE. Effect of long‐term treatment with an inhaled corticosteroid (budesonide) on airway hyperresponsiveness and clinical asthma in nonsteroid‐dependent asthmatics. American Review of Respiratory Disease 1990;142(4):832‐6. - PubMed
Karlberg 1993
-
- Karlberg J, Gelander L, Albertsson‐Wikland K. Distinctions between short‐ and long‐term human growth studies. Acta Paediatrics 1994;83:777‐8. - PubMed
Kelly 2012
Lai 2009
-
- Lai C, Beasley R, Crane J, Foliaki S, Shah J, Weiland S. Global variation in the prevalence and severity of asthma symptoms: Phase Three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009;64(6):476‐83. - PubMed
Leung 2003
-
- Leung DY, Bloom JW. Update on glucocorticoid action and resistance. Journal of Allergy and Clinical Immunology 2003;111(1):3‐22. - PubMed
Lougheed 2012
Masoli 2004
-
- Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004;59(5):469‐78. - PubMed
NHLBI 2007
-
- National Heart Lung and Blood Institute. Guidelines for the diagnosis and management of asthma (EPR‐3). www.nhlbi.nih.gov/guidelines/asthma (accessed 20 June 2011).
Olivieri 1997
-
- Olivieri D, Chetta A, Donno M, Bertorelli G, Casalini A, Pesci A, et al. Effect of short‐term treatment with low‐dose inhaled fluticasone propionate on airway inflammation and remodeling in mild asthma: a placebo‐controlled study. American Journal of Respiratory and Critical Care Medicine 1997;155(6):1864‐71. - PubMed
Pedersen 2001
-
- Pedersen S. Do inhaled corticosteroids inhibit growth in children?. Americian Journal of Respiratory and Critical Care Medicine 2001;164(4):521‐35. - PubMed
Price 2002
-
- Price J, Hindmarsh P, Hughes S, Efthimiou J. Evaluating the effects of asthma therapy on childhood growth: what can be learnt from the published literature?. European Respiratory Journal 2002;19:1179‐93. - PubMed
Pruteanu 2012
Review Manager 5 [Computer program]
-
- Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan) Version 5.0. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.
Salvatoni 2003
-
- Salvatoni A, Piantanida E, Nosetti L, Nespoli L. Inhaled corticosteroids in childhood asthma: long‐term effects on growth and adrenocortical function. Paediatric Drugs 2003;5(6):351‐61. - PubMed
Sharek 2000a
Sharek 2000b
-
- Sharek PJ, Bergman DA. The effect of inhaled steroids on the linear growth of children with asthma: a meta‐analysis. Pediatrics 2000;106:e8. - PubMed
Sizonenko 2002
-
- Sizonenko PC. Effects of inhaled or nasal glucocorticosteroids on adrenal function and growth. Journal of Pediatric Endocrinology and Metabolism 2002;15(1):5‐26. - PubMed
Sobande 2008
-
- Sobande PO, Kercsmar CM. Inhaled corticosteroids in asthma management. Respiratory Care 2008;53(5):625‐33. - PubMed
Stein 2004
-
- Stein RT, Martinez FD. Asthma phenotypes in childhood: lessons from epidemiological approach. Paediatric Respiratory Reviews 2004;5(2):155‐61. - PubMed
Suissa 2000
-
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low‐dose inhaled corticosteroids and the prevention of death from asthma. New England Journal of Medicine 2000;343(5):332‐6. - PubMed
Van Essen‐Zandvliet 1992
-
- Essen‐Zandvliet EE, Hughes MD, Waalkens HJ, Duiverman EJ, Pocock SJ, Kerrebijn KF. Effects of 22 months of treatment with inhaled corticosteroids and/or beta‐2‐agonists on lung function, airway responsiveness, and symptoms in children with asthma. The Dutch Chronic Non‐specific Lung Disease Study Group. American Review of Respiratory Disease 1992;146(3):547‐54. - PubMed
Van Rens 1999
Witzmann 2000
-
- Witzmann KB, Fink RJ. Inhaled corticosteroids in childhood asthma: growing concerns. Drugs.2000;59 2000;59(Suppl 1):9‐14. - PubMed
Wolthers 1993
Wolthers 1997
-
- Wolthers O, Hansen M, Juul A, Nielsen HK, Pedersen S. Knemometry, urine cortisol excretion and measures of the insulin‐like growth factor axis and collagen turnover in the assessment of systemic activity of inhaled corticosteroids. Pediatric Research 1997;41:44‐50. - PubMed
Wolthers 2001
-
- Wolthers OD. Inhaled corticosteroids and growth. Journal of Pediatric Endocrinology and Metabolism 2001;14(Suppl 6):1487‐90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

