Mechanisms underlying the exquisite sensitivity of Candida albicans to combinatorial cationic and oxidative stress that enhances the potent fungicidal activity of phagocytes
- PMID: 25028425
- PMCID: PMC4161263
- DOI: 10.1128/mBio.01334-14
Mechanisms underlying the exquisite sensitivity of Candida albicans to combinatorial cationic and oxidative stress that enhances the potent fungicidal activity of phagocytes
Abstract
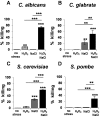
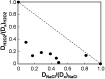
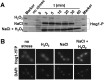
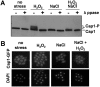
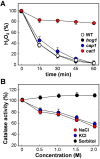
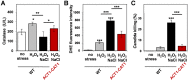
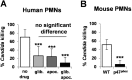
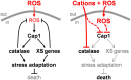
Immune cells exploit reactive oxygen species (ROS) and cationic fluxes to kill microbial pathogens, such as the fungus Candida albicans. Yet, C. albicans is resistant to these stresses in vitro. Therefore, what accounts for the potent antifungal activity of neutrophils? We show that simultaneous exposure to oxidative and cationic stresses is much more potent than the individual stresses themselves and that this combinatorial stress kills C. albicans synergistically in vitro. We also show that the high fungicidal activity of human neutrophils is dependent on the combinatorial effects of the oxidative burst and cationic fluxes, as their pharmacological attenuation with apocynin or glibenclamide reduced phagocytic potency to a similar extent. The mechanistic basis for the extreme potency of combinatorial cationic plus oxidative stress--a phenomenon we term stress pathway interference--lies with the inhibition of hydrogen peroxide detoxification by the cations. In C. albicans this causes the intracellular accumulation of ROS, the inhibition of Cap1 (a transcriptional activator that normally drives the transcriptional response to oxidative stress), and altered readouts of the stress-activated protein kinase Hog1. This leads to a loss of oxidative and cationic stress transcriptional outputs, a precipitous collapse in stress adaptation, and cell death. This stress pathway interference can be suppressed by ectopic catalase (Cat1) expression, which inhibits the intracellular accumulation of ROS and the synergistic killing of C. albicans cells by combinatorial cationic plus oxidative stress. Stress pathway interference represents a powerful fungicidal mechanism employed by the host that suggests novel approaches to potentiate antifungal therapy. Importance: The immune system combats infection via phagocytic cells that recognize and kill pathogenic microbes. Human neutrophils combat Candida infections by killing this fungus with a potent mix of chemicals that includes reactive oxygen species (ROS) and cations. Yet, Candida albicans is relatively resistant to these stresses in vitro. We show that it is the combination of oxidative plus cationic stresses that kills yeasts so effectively, and we define the molecular mechanisms that underlie this potency. Cations inhibit catalase. This leads to the accumulation of intracellular ROS and inhibits the transcription factor Cap1, which is critical for the oxidative stress response in C. albicans. This triggers a dramatic collapse in fungal stress adaptation and cell death. Blocking either the oxidative burst or cationic fluxes in human neutrophils significantly reduces their ability to kill this fungal pathogen, indicating that combinatorial stress is pivotal to immune surveillance.
Copyright © 2014 Kaloriti et al.
Figures

Comment in
-
Unrealistic nonphysiological amounts of reagents and a disregard for published literature.mBio. 2015 Apr 21;6(2):e00360-15. doi: 10.1128/mBio.00360-15. mBio. 2015. PMID: 25900656 Free PMC article. No abstract available.
-
Reply to "Unrealistic nonphysiological amounts of reagents and a disregard for published literature".mBio. 2015 Apr 21;6(2):e00450-15. doi: 10.1128/mBio.00450-15. mBio. 2015. PMID: 25900657 Free PMC article. No abstract available.
Similar articles
-
Mechanisms Underlying the Delayed Activation of the Cap1 Transcription Factor in Candida albicans following Combinatorial Oxidative and Cationic Stress Important for Phagocytic Potency.mBio. 2016 Mar 29;7(2):e00331. doi: 10.1128/mBio.00331-16. mBio. 2016. PMID: 27025253 Free PMC article.
-
Acidic/Alkaline Stress Mediates Responses to Azole Drugs and Oxidative Stress in Aspergillus fumigatus.Microbiol Spectr. 2022 Feb 23;10(1):e0199921. doi: 10.1128/spectrum.01999-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196814 Free PMC article.
-
Oxidative stress responses in the human fungal pathogen, Candida albicans.Biomolecules. 2015 Feb 25;5(1):142-65. doi: 10.3390/biom5010142. Biomolecules. 2015. PMID: 25723552 Free PMC article. Review.
-
Cellular responses of Candida albicans to phagocytosis and the extracellular activities of neutrophils are critical to counteract carbohydrate starvation, oxidative and nitrosative stress.PLoS One. 2012;7(12):e52850. doi: 10.1371/journal.pone.0052850. Epub 2012 Dec 21. PLoS One. 2012. PMID: 23285201 Free PMC article.
-
Stress adaptation in a pathogenic fungus.J Exp Biol. 2014 Jan 1;217(Pt 1):144-55. doi: 10.1242/jeb.088930. J Exp Biol. 2014. PMID: 24353214 Free PMC article. Review.
Cited by
-
Restoring glucose uptake rescues neutrophil dysfunction and protects against systemic fungal infection in mouse models of kidney disease.Sci Transl Med. 2020 Jun 17;12(548):eaay5691. doi: 10.1126/scitranslmed.aay5691. Sci Transl Med. 2020. PMID: 32554707 Free PMC article.
-
Protection of Candida parapsilosis from neutrophil killing through internalization by human endothelial cells.Virulence. 2015;6(5):504-14. doi: 10.1080/21505594.2015.1042643. Virulence. 2015. PMID: 26039751 Free PMC article.
-
Antifungal Properties of Cationic Phenylene Ethynylenes and Their Impact on β-Glucan Exposure.Antimicrob Agents Chemother. 2016 Jul 22;60(8):4519-29. doi: 10.1128/AAC.00317-16. Print 2016 Aug. Antimicrob Agents Chemother. 2016. PMID: 27161628 Free PMC article.
-
Mechanisms Underlying the Delayed Activation of the Cap1 Transcription Factor in Candida albicans following Combinatorial Oxidative and Cationic Stress Important for Phagocytic Potency.mBio. 2016 Mar 29;7(2):e00331. doi: 10.1128/mBio.00331-16. mBio. 2016. PMID: 27025253 Free PMC article.
-
Cell biology of Candida albicans-host interactions.Curr Opin Microbiol. 2016 Dec;34:111-118. doi: 10.1016/j.mib.2016.08.006. Epub 2016 Sep 28. Curr Opin Microbiol. 2016. PMID: 27689902 Free PMC article. Review.
References
-
- Odds FC. 1988. Candida and Candidiasis. London, United Kingdom
-
- Calderone RA, Clancy CJ. 2011. Candida and candidiasis. ASM Press, Washington, DC
Publication types
MeSH terms
Substances
Grants and funding
- 097377/WT_/Wellcome Trust/United Kingdom
- R01 AI079253/AI/NIAID NIH HHS/United States
- 089930/WT_/Wellcome Trust/United Kingdom
- BB/F005210/1-2/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 086048/WT_/Wellcome Trust/United Kingdom
- BB/F00513X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- CA016056/CA/NCI NIH HHS/United States
- 101873/WT_/Wellcome Trust/United Kingdom
- 249793/ERC_/European Research Council/International
- P30 CA016056/CA/NCI NIH HHS/United States
- R01AI079253/AI/NIAID NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- BB/F005210/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 080088/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
