Increased sensitivity to ionizing radiation by targeting the homologous recombination pathway in glioma initiating cells
- PMID: 25017126
- PMCID: PMC5528585
- DOI: 10.1016/j.molonc.2014.06.012
Increased sensitivity to ionizing radiation by targeting the homologous recombination pathway in glioma initiating cells
Abstract
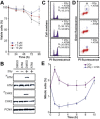
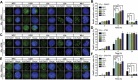
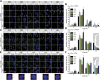
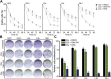
Glioblastoma is deemed the most malignant form of brain tumour, particularly due to its resistance to conventional treatments. A small surviving group of aberrant stem cells termed glioma initiation cells (GICs) that escape surgical debulking are suggested to be the cause of this resistance. Relatively quiescent in nature, GICs are capable of driving tumour recurrence and undergo lineage differentiation. Most importantly, these GICs are resistant to radiotherapy, suggesting that radioresistance contribute to their survival. In a previous study, we demonstrated that GICs had a restricted double strand break (DSB) repair pathway involving predominantly homologous recombination (HR) associated with a lack of functional G1/S checkpoint arrest. This unusual behaviour led to less efficient non-homologous end joining (NHEJ) repair and overall slower DNA DSB repair kinetics. To determine whether specific targeting of the HR pathway with small molecule inhibitors could increase GIC radiosensitivity, we used the Ataxia-telangiectasia mutated inhibitor (ATMi) to ablate HR and the DNA-dependent protein kinase inhibitor (DNA-PKi) to inhibit NHEJ. Pre-treatment with ATMi prior to ionizing radiation (IR) exposure prevented HR-mediated DNA DSB repair as measured by Rad51 foci accumulation. Increased cell death in vitro and improved in vivo animal survival could be observed with combined ATMi and IR treatment. Conversely, DNA-PKi treatment had minimal impact on GICs ability to resolve DNA DSB after IR with only partial reduction in cell survival, confirming the major role of HR. These results provide a mechanistic insight into the predominant form of DNA DSB repair in GICs, which when targeted may be a potential translational approach to increase patient survival.
Keywords: ATM inhibitor; DNA damage; DNA double strand break repair; Glioma initiating cell; Homologous recombination; Neural progenitor cell.
Copyright © 2014. Published by Elsevier B.V.
Figures

Similar articles
-
Regulation of ATM in DNA double strand break repair accounts for the radiosensitivity in human cells exposed to high linear energy transfer ionizing radiation.Mutat Res. 2009 Nov 2;670(1-2):15-23. doi: 10.1016/j.mrfmmm.2009.06.016. Epub 2009 Jul 5. Mutat Res. 2009. PMID: 19583974
-
PAXX and XLF DNA repair factors are functionally redundant in joining DNA breaks in a G1-arrested progenitor B-cell line.Proc Natl Acad Sci U S A. 2016 Sep 20;113(38):10619-24. doi: 10.1073/pnas.1611882113. Epub 2016 Sep 6. Proc Natl Acad Sci U S A. 2016. PMID: 27601633 Free PMC article.
-
Dynamic dependence on ATR and ATM for double-strand break repair in human embryonic stem cells and neural descendants.PLoS One. 2010 Apr 2;5(4):e10001. doi: 10.1371/journal.pone.0010001. PLoS One. 2010. PMID: 20368801 Free PMC article.
-
Roles for the DNA-PK complex and 53BP1 in protecting ends from resection during DNA double-strand break repair.J Radiat Res. 2020 Sep 8;61(5):718-726. doi: 10.1093/jrr/rraa053. J Radiat Res. 2020. PMID: 32779701 Free PMC article. Review.
-
Roles for 53BP1 in the repair of radiation-induced DNA double strand breaks.DNA Repair (Amst). 2020 Sep;93:102915. doi: 10.1016/j.dnarep.2020.102915. DNA Repair (Amst). 2020. PMID: 33087281 Review.
Cited by
-
Myosin Heavy Chain 10 (MYH10) Gene Silencing Reduces Cell Migration and Invasion in the Glioma Cell Lines U251, T98G, and SHG44 by Inhibiting the Wnt/β-Catenin Pathway.Med Sci Monit. 2018 Dec 15;24:9110-9119. doi: 10.12659/MSM.911523. Med Sci Monit. 2018. PMID: 30552850 Free PMC article.
-
Reparative properties of human glioblastoma cells after single exposure to a wide range of X-ray doses.Front Oncol. 2022 Aug 4;12:912741. doi: 10.3389/fonc.2022.912741. eCollection 2022. Front Oncol. 2022. PMID: 35992802 Free PMC article.
-
Hyperthermia Sensitizes Glioma Stem-like Cells to Radiation by Inhibiting AKT Signaling.Cancer Res. 2015 Apr 15;75(8):1760-9. doi: 10.1158/0008-5472.CAN-14-3621. Epub 2015 Feb 20. Cancer Res. 2015. PMID: 25712125 Free PMC article.
-
Activation of the Unfolded Protein Response via Inhibition of Protein Disulfide Isomerase Decreases the Capacity for DNA Repair to Sensitize Glioblastoma to Radiotherapy.Cancer Res. 2019 Jun 1;79(11):2923-2932. doi: 10.1158/0008-5472.CAN-18-2540. Epub 2019 Apr 17. Cancer Res. 2019. PMID: 30996048 Free PMC article.
-
MiR-210 up-regulation inhibits proliferation and induces apoptosis in glioma cells by targeting SIN3A.Med Sci Monit. 2014 Dec 7;20:2571-7. doi: 10.12659/MSM.892994. Med Sci Monit. 2014. PMID: 25481483 Free PMC article.
References
-
- Allen, C. , Halbrook, J. , Nickoloff, J.A. , 2003. Interactive competition between homologous recombination and non-homologous end joining. Mol. Cancer Res.. 1, 913–920. - PubMed
-
- Bao, S. , Wu, Q. , McLendon, R.E. , Hao, Y. , Shi, Q. , Hjelmeland, A.B. , Dewhirst, M.W. , Bigner, D.D. , Rich, J.N. , 2006. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 444, 756–760. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

