Ugi 4-CR Synthesis of γ- and δ-Lactams providing new access to diverse enzyme interactions, a PDB analysis
- PMID: 25013719
- PMCID: PMC4084911
- DOI: 10.1039/C4MD00162A
Ugi 4-CR Synthesis of γ- and δ-Lactams providing new access to diverse enzyme interactions, a PDB analysis
Abstract
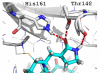
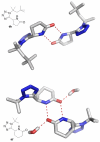
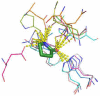
A three step synthesis of N-unsubstituted tetrazolo γ- and δ-lactams involving a key Ugi-4CR is presented. The compounds, otherwise difficult to access, are conveniently synthesized in overall good yields by our route. PDB analysis of the N-unsubstituted γ- and δ-lactam fragment reveals a strongly tri-directional hydrogen bond donor acceptor interaction with the amino acids of the binding sites.
Figures

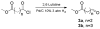
Similar articles
-
Ammonia synthons for the multicomponent assembly of complex gamma-lactams.Proc Natl Acad Sci U S A. 2011 Apr 26;108(17):6781-6. doi: 10.1073/pnas.1015261108. Epub 2011 Feb 2. Proc Natl Acad Sci U S A. 2011. PMID: 21289284 Free PMC article.
-
An efficient microwave-assisted synthesis of cotinine and iso-cotinine analogs from an Ugi-4CR approach.Org Biomol Chem. 2015 Sep 14;13(34):9065-71. doi: 10.1039/c5ob01170a. Epub 2015 Jul 29. Org Biomol Chem. 2015. PMID: 26220292
-
Multicomponent Approach to a Library of N-Substituted γ-Lactams.ACS Comb Sci. 2019 Jan 14;21(1):28-34. doi: 10.1021/acscombsci.8b00147. Epub 2018 Dec 24. ACS Comb Sci. 2019. PMID: 30563326
-
Synthesis of N-heterocycles containing 1,5-disubstituted-1H-tetrazole via post-Ugi-azide reaction.Mol Divers. 2020 Aug;24(3):841-853. doi: 10.1007/s11030-019-09972-1. Epub 2019 Jun 20. Mol Divers. 2020. PMID: 31222498 Review.
-
Synthesis of highly functionalized organic compounds through Ugi post-transformations started from propiolic acids.Mol Divers. 2020 Aug;24(3):855-887. doi: 10.1007/s11030-019-09975-y. Epub 2019 Jul 19. Mol Divers. 2020. PMID: 31325081 Review.
Cited by
-
Diverse Isoquinoline Scaffolds by Ugi/Pomeranz-Fritsch and Ugi/Schlittler-Müller Reactions.Org Lett. 2019 May 17;21(10):3533-3537. doi: 10.1021/acs.orglett.9b00778. Epub 2019 Apr 29. Org Lett. 2019. PMID: 31033297 Free PMC article.
-
Tetrazoles via Multicomponent Reactions.Chem Rev. 2019 Feb 13;119(3):1970-2042. doi: 10.1021/acs.chemrev.8b00564. Epub 2019 Feb 1. Chem Rev. 2019. PMID: 30707567 Free PMC article. Review.
-
Stefano Marcaccini: a pioneer in isocyanide chemistry.Mol Divers. 2024 Feb;28(1):335-418. doi: 10.1007/s11030-023-10641-7. Epub 2023 Apr 12. Mol Divers. 2024. PMID: 37043161 Free PMC article. Review.
References
-
- Dömling A. Chem. Rev. 2006;106:17. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

