Genogroup IV and VI canine noroviruses interact with histo-blood group antigens
- PMID: 25008923
- PMCID: PMC4178834
- DOI: 10.1128/JVI.01008-14
Genogroup IV and VI canine noroviruses interact with histo-blood group antigens
Abstract
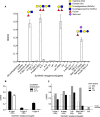
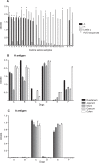
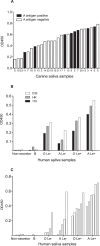
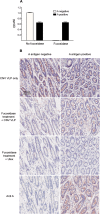
Human noroviruses (HuNV) are a significant cause of viral gastroenteritis in humans worldwide. HuNV attaches to cell surface carbohydrate structures known as histo-blood group antigens (HBGAs) prior to internalization, and HBGA polymorphism among human populations is closely linked to susceptibility to HuNV. Noroviruses are divided into 6 genogroups, with human strains grouped into genogroups I (GI), II, and IV. Canine norovirus (CNV) is a recently discovered pathogen in dogs, with strains classified into genogroups IV and VI. Whereas it is known that GI to GIII noroviruses bind to HBGAs and GV noroviruses recognize terminal sialic acid residues, the attachment factors for GIV and GVI noroviruses have not been reported. This study sought to determine the carbohydrate binding specificity of CNV and to compare it to the binding specificities of noroviruses from other genogroups. A panel of synthetic oligosaccharides were used to assess the binding specificity of CNV virus-like particles (VLPs) and identified α1,2-fucose as a key attachment factor. CNV VLP binding to canine saliva and tissue samples using enzyme-linked immunosorbent assays (ELISAs) and immunohistochemistry confirmed that α1,2-fucose-containing H and A antigens of the HBGA family were recognized by CNV. Phenotyping studies demonstrated expression of these antigens in a population of dogs. The virus-ligand interaction was further characterized using blockade studies, cell lines expressing HBGAs, and enzymatic removal of candidate carbohydrates from tissue sections. Recognition of HBGAs by CNV provides new insights into the evolution of noroviruses and raises concerns regarding the potential for zoonotic transmission of CNV to humans.
Importance: Infections with human norovirus cause acute gastroenteritis in millions of people each year worldwide. Noroviruses can also affect nonhuman species and are divided into 6 different groups based on their capsid sequences. Human noroviruses in genogroups I and II interact with histo-blood group antigen carbohydrates, bovine noroviruses (genogroup III) interact with alpha-galactosidase (α-Gal) carbohydrates, and murine norovirus (genogroup V) recognizes sialic acids. The canine-specific strains of norovirus are grouped into genogroups IV and VI, and this study is the first to characterize which carbohydrate structures they can recognize. Using canine norovirus virus-like particles, this work shows that representative genogroup IV and VI viruses can interact with histo-blood group antigens. The binding specificity of canine noroviruses is therefore very similar to that of the human norovirus strains classified into genogroups I and II. This raises interesting questions about the evolution of noroviruses and suggests it may be possible for canine norovirus to infect humans.
Copyright © 2014 Caddy et al.
Figures




Similar articles
-
Bovine Nebovirus Interacts with a Wide Spectrum of Histo-Blood Group Antigens.J Virol. 2018 Apr 13;92(9):e02160-17. doi: 10.1128/JVI.02160-17. Print 2018 May 1. J Virol. 2018. PMID: 29467317 Free PMC article.
-
Crystal structures of GII.10 and GII.12 norovirus protruding domains in complex with histo-blood group antigens reveal details for a potential site of vulnerability.J Virol. 2011 Jul;85(13):6687-701. doi: 10.1128/JVI.00246-11. Epub 2011 Apr 27. J Virol. 2011. PMID: 21525337 Free PMC article.
-
Structural analysis of determinants of histo-blood group antigen binding specificity in genogroup I noroviruses.J Virol. 2014 Jun;88(11):6168-80. doi: 10.1128/JVI.00201-14. Epub 2014 Mar 19. J Virol. 2014. PMID: 24648450 Free PMC article.
-
[Noroviruses--tactic of spread].Przegl Epidemiol. 2009;63(1):5-9. Przegl Epidemiol. 2009. PMID: 19522218 Review. Polish.
-
Human Norovirus Interactions with Histo-Blood Group Antigens and Human Milk Oligosaccharides.J Virol. 2016 Jun 10;90(13):5855-5859. doi: 10.1128/JVI.00317-16. Print 2016 Jul 1. J Virol. 2016. PMID: 27122582 Free PMC article. Review.
Cited by
-
Microbial lectome versus host glycolipidome: How pathogens exploit glycosphingolipids to invade, dupe or kill.Front Microbiol. 2022 Aug 19;13:958653. doi: 10.3389/fmicb.2022.958653. eCollection 2022. Front Microbiol. 2022. PMID: 36060781 Free PMC article. Review.
-
Genomics Analyses of GIV and GVI Noroviruses Reveal the Distinct Clustering of Human and Animal Viruses.Viruses. 2019 Mar 1;11(3):204. doi: 10.3390/v11030204. Viruses. 2019. PMID: 30823663 Free PMC article.
-
Norovirus Attachment and Entry.Viruses. 2019 May 30;11(6):495. doi: 10.3390/v11060495. Viruses. 2019. PMID: 31151248 Free PMC article. Review.
-
Evidence for human norovirus infection of dogs in the United kingdom.J Clin Microbiol. 2015 Jun;53(6):1873-83. doi: 10.1128/JCM.02778-14. Epub 2015 Apr 1. J Clin Microbiol. 2015. PMID: 25832298 Free PMC article.
-
Feline Virome-A Review of Novel Enteric Viruses Detected in Cats.Viruses. 2019 Sep 30;11(10):908. doi: 10.3390/v11100908. Viruses. 2019. PMID: 31575055 Free PMC article. Review.
References
-
- Tam C, Rodrigues L, Viviani L, Dodds J, Evans Hunter P, Gray J, Letley L, Rait G, Tompkins D, O'Brien S. 2012. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut 61:69–77. 10.1136/gut.2011.238386 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

