Envelope glycoprotein binding to the integrin α4β7 is not a general property of most HIV-1 strains
- PMID: 25008916
- PMCID: PMC4178844
- DOI: 10.1128/JVI.03296-13
Envelope glycoprotein binding to the integrin α4β7 is not a general property of most HIV-1 strains
Abstract
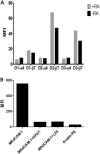
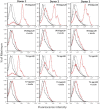
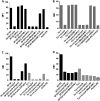
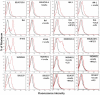
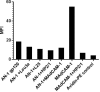
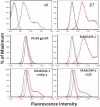
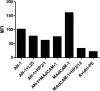
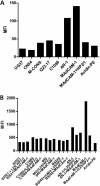
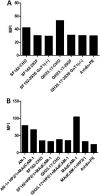
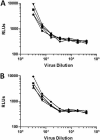
The HIV-1 surface glycoprotein gp120 has been reported to bind and signal through α4β7 by means of a tripeptide motif in the V2 loop that mimics structures present in the natural ligands for α4β7, suggesting that α4β7 may facilitate HIV-1 infection of CD4(+) T cells in the gut. Furthermore, immune correlates in the RV144 vaccine efficacy trial generated the hypothesis that V1V2 antibodies to an epitope near the putative α4β7 binding motif may play a role in protection against HIV-1 infection. In the interest of developing an assay to detect antibodies that block gp120 binding to α4β7, we used retinoic acid (RA)-activated human peripheral blood mononuclear cells (PBMCs) and transfected HEK293T (293T) cells expressing the integrin complex to study the α4β7 binding properties of 16 HIV-1 envelope glycoproteins. The natural ligand for α4β7, mucosal addressin cell adhesion molecule-1 (MAdCAM-1), bound efficiently to RA-activated PBMCs and transfected 293T cells, and this binding was blocked by antibodies to α4. gp120 from multiple HIV-1 subtypes bound to RA-activated PBMCs from three donors in a CD4-dependent manner, but little or no α4β7 binding was detected. Similarly, little or no binding to α4β7 on transfected 293T cells was detected with multiple gp120s and gp140s, including gp120s from transmitted/founder strains, or when gp120 was produced in CHO, 293T, and 293S/GnT1(-/-) cells. Finally, we found no evidence that infectious HIV-1 virions produced in either PBMCs or 293T cells could bind α4β7 on transfected 293T cells. Infectious HIV-1 virions and most gp120s/gp140s appear to be poor ligands for the α4β7 integrin complex under the conditions tested here.
Importance: Certain HIV-1 gp120 envelope glycoproteins have been shown to bind the gut-homing receptor α4β7, and it has been suggested that this binding facilitates mucosal transmission and virus replication in the gut mucosa. Additional evidence has generated the hypothesis that antibodies that bind near the putative α4β7 binding motif in the V2 loop of gp120, possibly disrupting gp120-α4β7 binding, may be important for HIV-1 vaccines. Our evidence indicates that infectious HIV-1 virions and many gp120s lack detectable α4β7 binding activity, suggesting that this homing receptor may play a limited role in direct HIV-1 infection of cells.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Extracellular Matrix Proteins Mediate HIV-1 gp120 Interactions with α4β7.J Virol. 2017 Oct 13;91(21):e01005-17. doi: 10.1128/JVI.01005-17. Print 2017 Nov 1. J Virol. 2017. PMID: 28814519 Free PMC article.
-
Identification of New Regions in HIV-1 gp120 Variable 2 and 3 Loops that Bind to α4β7 Integrin Receptor.PLoS One. 2015 Dec 1;10(12):e0143895. doi: 10.1371/journal.pone.0143895. eCollection 2015. PLoS One. 2015. PMID: 26625359 Free PMC article. Clinical Trial.
-
Select gp120 V2 domain specific antibodies derived from HIV and SIV infection and vaccination inhibit gp120 binding to α4β7.PLoS Pathog. 2018 Aug 28;14(8):e1007278. doi: 10.1371/journal.ppat.1007278. eCollection 2018 Aug. PLoS Pathog. 2018. PMID: 30153309 Free PMC article.
-
HIV-1 envelope, integrins and co-receptor use in mucosal transmission of HIV.J Transl Med. 2011 Jan 27;9 Suppl 1(Suppl 1):S2. doi: 10.1186/1479-5876-9-S1-S2. J Transl Med. 2011. PMID: 21284901 Free PMC article. Review.
-
Integrin α4β7 in HIV-1 infection: A critical review.J Leukoc Biol. 2020 Aug;108(2):627-632. doi: 10.1002/JLB.4MR0120-208R. Epub 2020 Apr 9. J Leukoc Biol. 2020. PMID: 32272507 Review.
Cited by
-
Integrin α4β7 expression on peripheral blood CD4+ T cells predicts HIV acquisition and disease progression outcomes.Sci Transl Med. 2018 Jan 24;10(425):eaam6354. doi: 10.1126/scitranslmed.aam6354. Sci Transl Med. 2018. PMID: 29367348 Free PMC article.
-
Differentiating founder and chronic HIV envelope sequences.PLoS One. 2017 Feb 10;12(2):e0171572. doi: 10.1371/journal.pone.0171572. eCollection 2017. PLoS One. 2017. PMID: 28187204 Free PMC article.
-
Glycosylation and oligomeric state of envelope protein might influence HIV-1 virion capture by α4β7 integrin.Virology. 2017 Aug;508:199-212. doi: 10.1016/j.virol.2017.05.016. Epub 2017 May 31. Virology. 2017. PMID: 28577856 Free PMC article.
-
Extracellular Matrix Proteins Mediate HIV-1 gp120 Interactions with α4β7.J Virol. 2017 Oct 13;91(21):e01005-17. doi: 10.1128/JVI.01005-17. Print 2017 Nov 1. J Virol. 2017. PMID: 28814519 Free PMC article.
-
Bottlenecks in HIV-1 transmission: insights from the study of founder viruses.Nat Rev Microbiol. 2015 Jul;13(7):414-25. doi: 10.1038/nrmicro3471. Epub 2015 Jun 8. Nat Rev Microbiol. 2015. PMID: 26052661 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

