Quantitative proteomic analysis reveals metabolic alterations, calcium dysregulation, and increased expression of extracellular matrix proteins in laminin α2 chain-deficient muscle
- PMID: 24994560
- PMCID: PMC4223487
- DOI: 10.1074/mcp.M113.032276
Quantitative proteomic analysis reveals metabolic alterations, calcium dysregulation, and increased expression of extracellular matrix proteins in laminin α2 chain-deficient muscle
Abstract
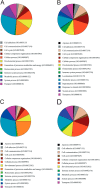
Congenital muscular dystrophy with laminin α2 chain deficiency (MDC1A) is one of the most severe forms of muscular disease and is characterized by severe muscle weakness and delayed motor milestones. The genetic basis of MDC1A is well known, yet the secondary mechanisms ultimately leading to muscle degeneration and subsequent connective tissue infiltration are not fully understood. In order to obtain new insights into the molecular mechanisms underlying MDC1A, we performed a comparative proteomic analysis of affected muscles (diaphragm and gastrocnemius) from laminin α2 chain-deficient dy(3K)/dy(3K) mice, using multidimensional protein identification technology combined with tandem mass tags. Out of the approximately 700 identified proteins, 113 and 101 proteins, respectively, were differentially expressed in the diseased gastrocnemius and diaphragm muscles compared with normal muscles. A large portion of these proteins are involved in different metabolic processes, bind calcium, or are expressed in the extracellular matrix. Our findings suggest that metabolic alterations and calcium dysregulation could be novel mechanisms that underlie MDC1A and might be targets that should be explored for therapy. Also, detailed knowledge of the composition of fibrotic tissue, rich in extracellular matrix proteins, in laminin α2 chain-deficient muscle might help in the design of future anti-fibrotic treatments. All MS data have been deposited in the ProteomeXchange with identifier PXD000978 (http://proteomecentral.proteomexchange.org/dataset/PXD000978).
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Potent pro-inflammatory and pro-fibrotic molecules, osteopontin and galectin-3, are not major disease modulators of laminin α2 chain-deficient muscular dystrophy.Sci Rep. 2017 Mar 10;7:44059. doi: 10.1038/srep44059. Sci Rep. 2017. PMID: 28281577 Free PMC article.
-
Proteasome inhibition improves the muscle of laminin α2 chain-deficient mice.Hum Mol Genet. 2011 Feb 1;20(3):541-52. doi: 10.1093/hmg/ddq499. Epub 2010 Nov 17. Hum Mol Genet. 2011. PMID: 21084425
-
Exon Skipping Using Antisense Oligonucleotides for Laminin-Alpha2-Deficient Muscular Dystrophy.Methods Mol Biol. 2018;1828:553-564. doi: 10.1007/978-1-4939-8651-4_36. Methods Mol Biol. 2018. PMID: 30171567
-
Laminin-α2 Chain-Deficient Congenital Muscular Dystrophy: Pathophysiology and Development of Treatment.Curr Top Membr. 2015;76:31-60. doi: 10.1016/bs.ctm.2015.05.002. Curr Top Membr. 2015. PMID: 26610911 Review.
-
Merosin and congenital muscular dystrophy.Microsc Res Tech. 2000 Feb 1-15;48(3-4):181-91. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<181::AID-JEMT6>3.0.CO;2-Q. Microsc Res Tech. 2000. PMID: 10679965 Review.
Cited by
-
Effects of metformin on congenital muscular dystrophy type 1A disease progression in mice: a gender impact study.Sci Rep. 2018 Nov 2;8(1):16302. doi: 10.1038/s41598-018-34362-2. Sci Rep. 2018. PMID: 30389963 Free PMC article.
-
Merosin deficient congenital muscular dystrophy type 1A: An international workshop on the road to therapy 15-17 November 2019, Maastricht, the Netherlands.Neuromuscul Disord. 2021 Jul;31(7):673-680. doi: 10.1016/j.nmd.2021.04.003. Epub 2021 May 1. Neuromuscul Disord. 2021. PMID: 34130888 Free PMC article. No abstract available.
-
Custom 4-Plex DiLeu Isobaric Labels Enable Relative Quantification of Urinary Proteins in Men with Lower Urinary Tract Symptoms (LUTS).PLoS One. 2015 Aug 12;10(8):e0135415. doi: 10.1371/journal.pone.0135415. eCollection 2015. PLoS One. 2015. PMID: 26267142 Free PMC article.
-
Antioxidants Reduce Muscular Dystrophy in the dy2J/dy2J Mouse Model of Laminin α2 Chain-Deficient Muscular Dystrophy.Antioxidants (Basel). 2020 Mar 18;9(3):244. doi: 10.3390/antiox9030244. Antioxidants (Basel). 2020. PMID: 32197453 Free PMC article.
-
At the Crossroads of Clinical and Preclinical Research for Muscular Dystrophy-Are We Closer to Effective Treatment for Patients?Int J Mol Sci. 2018 May 16;19(5):1490. doi: 10.3390/ijms19051490. Int J Mol Sci. 2018. PMID: 29772730 Free PMC article. Review.
References
-
- Allamand V., Guicheney P. (2002) Merosin-deficient muscular dystrophy, autosomal recessive (MDC1A, MIM#156225, LAMA2 gene coding for α2 chain of laminin). Eur. J. Hum. Genet. 10, 91–94 - PubMed
-
- Helbling-Leclerc A., Zhang X., Topaloglu H., Cruaud C., Tesson F., Weissenbach J., Tomé F. M. S., Schwartz K., Fardeau M., Tryggvason K., Guicheney P. (1995) Mutations in the laminin α2 chain gene (LAMA2) cause merosin-deficient muscular dystrophy. Nat. Genet. 11, 216–218 - PubMed
-
- Voit T., Tomé F. S. (2004) The congenital muscular dystrophies. In Myology (Angel A., Franzini-Armstrong C., eds) Vol. 2, pp. 1203–1238, McGraw-Hill, New York
-
- Ibraghimov-Beskrovnaya O., Ervasti J. M., Leveille C. J., Slaughter C. A., Sernett S. W., Campbell K. P. (1992) Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 355, 696–702 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

