Systemic hematogenous maintenance of memory inflation by MCMV infection
- PMID: 24992722
- PMCID: PMC4081724
- DOI: 10.1371/journal.ppat.1004233
Systemic hematogenous maintenance of memory inflation by MCMV infection
Abstract
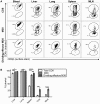
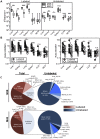
Several low-grade persistent viral infections induce and sustain very large numbers of virus-specific effector T cells. This was first described as a response to cytomegalovirus (CMV), a herpesvirus that establishes a life-long persistent/latent infection, and sustains the largest known effector T cell populations in healthy people. These T cells remain functional and traffic systemically, which has led to the recent exploration of CMV as a persistent vaccine vector. However, the maintenance of this remarkable response is not understood. Current models propose that reservoirs of viral antigen and/or latently infected cells in lymph nodes stimulate T cell proliferation and effector differentiation, followed by migration of progeny to non-lymphoid tissues where they control CMV reactivation. We tested this model using murine CMV (MCMV), a natural mouse pathogen and homologue of human CMV (HCMV). While T cells within draining lymph nodes divided at a higher rate than cells elsewhere, antigen-dependent proliferation of MCMV-specific effector T cells was observed systemically. Strikingly, inhibition of T cell egress from lymph nodes failed to eliminate systemic T cell division, and did not prevent the maintenance of the inflationary populations. In fact, we found that the vast majority of inflationary cells, including most cells undergoing antigen-driven division, had not migrated into the parenchyma of non-lymphoid tissues but were instead exposed to the blood supply. Indeed, the immunodominance and effector phenotype of inflationary cells, both of which are primary hallmarks of memory inflation, were largely confined to blood-localized T cells. Together these results support a new model of MCMV-driven memory inflation in which most immune surveillance occurs in circulation, and in which most inflationary effector T cells are produced in response to viral antigen presented by cells that are accessible to the blood supply.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Sustained CD8+ T cell memory inflation after infection with a single-cycle cytomegalovirus.PLoS Pathog. 2011 Oct;7(10):e1002295. doi: 10.1371/journal.ppat.1002295. Epub 2011 Oct 6. PLoS Pathog. 2011. PMID: 21998590 Free PMC article.
-
Stochastic Episodes of Latent Cytomegalovirus Transcription Drive CD8 T-Cell "Memory Inflation" and Avoid Immune Evasion.Front Immunol. 2021 Apr 22;12:668885. doi: 10.3389/fimmu.2021.668885. eCollection 2021. Front Immunol. 2021. PMID: 33968074 Free PMC article.
-
Investigating the Dynamics of MCMV-Specific CD8+ T Cell Responses in Individual Hosts.Front Immunol. 2019 Jun 19;10:1358. doi: 10.3389/fimmu.2019.01358. eCollection 2019. Front Immunol. 2019. PMID: 31281313 Free PMC article.
-
Fuel and brake of memory T cell inflation.Med Microbiol Immunol. 2019 Aug;208(3-4):329-338. doi: 10.1007/s00430-019-00587-9. Epub 2019 Mar 9. Med Microbiol Immunol. 2019. PMID: 30852648 Review.
-
Dendritic cells in cytomegalovirus infection: viral evasion and host countermeasures.APMIS. 2009 May;117(5-6):413-26. doi: 10.1111/j.1600-0463.2009.02449.x. APMIS. 2009. PMID: 19400865 Review.
Cited by
-
Persistent viral replication and the development of T-cell responses after intranasal infection by MCMV.Med Microbiol Immunol. 2019 Aug;208(3-4):457-468. doi: 10.1007/s00430-019-00589-7. Epub 2019 Mar 8. Med Microbiol Immunol. 2019. PMID: 30848361 Free PMC article.
-
Mucosal T-cell responses to chronic viral infections: Implications for vaccine design.Cell Mol Immunol. 2024 Sep;21(9):982-998. doi: 10.1038/s41423-024-01140-2. Epub 2024 Mar 8. Cell Mol Immunol. 2024. PMID: 38459243 Free PMC article. Review.
-
From Vaccine Vector to Oncomodulation: Understanding the Complex Interplay between CMV and Cancer.Vaccines (Basel). 2019 Jul 9;7(3):62. doi: 10.3390/vaccines7030062. Vaccines (Basel). 2019. PMID: 31323930 Free PMC article. Review.
-
Advances in cytomegalovirus (CMV) biology and its relationship to health, diseases, and aging.Geroscience. 2020 Apr;42(2):495-504. doi: 10.1007/s11357-020-00170-8. Epub 2020 Mar 11. Geroscience. 2020. PMID: 32162210 Free PMC article. Review.
-
Exploring the Potential of Cytomegalovirus-Based Vectors: A Review.Viruses. 2023 Oct 2;15(10):2043. doi: 10.3390/v15102043. Viruses. 2023. PMID: 37896820 Free PMC article. Review.
References
-
- Jarvis MA, Nelson JA (2002) Human cytomegalovirus persistence and latency in endothelial cells and macrophages. Curr Opin Microbiol 5: 403–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

