Store-operated CRAC channels regulate gene expression and proliferation in neural progenitor cells
- PMID: 24990931
- PMCID: PMC4078087
- DOI: 10.1523/JNEUROSCI.0263-14.2014
Store-operated CRAC channels regulate gene expression and proliferation in neural progenitor cells
Abstract
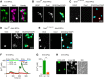
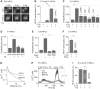
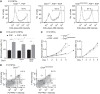
Calcium signals regulate many critical processes during vertebrate brain development including neurogenesis, neurotransmitter specification, and axonal outgrowth. However, the identity of the ion channels mediating Ca(2+) signaling in the developing nervous system is not well defined. Here, we report that embryonic and adult mouse neural stem/progenitor cells (NSCs/NPCs) exhibit store-operated Ca(2+) entry (SOCE) mediated by Ca(2+) release-activated Ca(2+) (CRAC) channels. SOCE in NPCs was blocked by the CRAC channel inhibitors La(3+), BTP2, and 2-APB and Western blots revealed the presence of the canonical CRAC channel proteins STIM1 and Orai1. Knock down of STIM1 or Orai1 significantly diminished SOCE in NPCs, and SOCE was lost in NPCs from transgenic mice lacking Orai1 or STIM1 and in knock-in mice expressing the loss-of-function Orai1 mutant, R93W. Therefore, STIM1 and Orai1 make essential contributions to SOCE in NPCs. SOCE in NPCs was activated by epidermal growth factor and acetylcholine, the latter occurring through muscarinic receptors. Activation of SOCE stimulated gene transcription through calcineurin/NFAT (nuclear factor of activated T cells) signaling through a mechanism consistent with local Ca(2+) signaling by Ca(2+) microdomains near CRAC channels. Importantly, suppression or deletion of STIM1 and Orai1 expression significantly attenuated proliferation of embryonic and adult NPCs cultured as neurospheres and, in vivo, in the subventricular zone of adult mice. These findings show that CRAC channels serve as a major route of Ca(2+) entry in NPCs and regulate key effector functions including gene expression and proliferation, indicating that CRAC channels are important regulators of mammalian neurogenesis.
Keywords: CRAC channels; Orai1; STIM1; calcium; progenitor cell; proliferation.
Copyright © 2014 the authors 0270-6474/14/349107-17$15.00/0.
Figures






Similar articles
-
Local Ca²+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca²+ signals required for specific cell functions.PLoS Biol. 2011 Mar;9(3):e1001025. doi: 10.1371/journal.pbio.1001025. Epub 2011 Mar 8. PLoS Biol. 2011. PMID: 21408196 Free PMC article.
-
Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation.Circ Res. 2008 Nov 21;103(11):1289-99. doi: 10.1161/01.RES.0000338496.95579.56. Epub 2008 Oct 9. Circ Res. 2008. PMID: 18845811 Free PMC article.
-
Store-Independent Orai Channels Regulated by STIM.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. PMID: 30299650 Free Books & Documents. Review.
-
Airway smooth muscle STIM1 and Orai1 are upregulated in asthmatic mice and mediate PDGF-activated SOCE, CRAC currents, proliferation, and migration.Pflugers Arch. 2012 Nov;464(5):481-92. doi: 10.1007/s00424-012-1160-5. Epub 2012 Sep 27. Pflugers Arch. 2012. PMID: 23014880 Free PMC article.
-
Modulation of Orai1 and STIM1 by Cellular Factors.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 4. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 4. PMID: 30299655 Free Books & Documents. Review.
Cited by
-
NEUROD2 Regulates Stim1 Expression and Store-Operated Calcium Entry in Cortical Neurons.eNeuro. 2017 Mar 9;4(1):ENEURO.0255-16.2017. doi: 10.1523/ENEURO.0255-16.2017. eCollection 2017 Jan-Feb. eNeuro. 2017. PMID: 28303257 Free PMC article.
-
Distinct spatial Ca2+ signatures selectively activate different NFAT transcription factor isoforms.Mol Cell. 2015 Apr 16;58(2):232-43. doi: 10.1016/j.molcel.2015.02.027. Epub 2015 Mar 26. Mol Cell. 2015. PMID: 25818645 Free PMC article.
-
Adult neural progenitor cells from Huntington's disease mouse brain exhibit increased proliferation and migration due to enhanced calcium and ROS signals.Cell Prolif. 2015 Oct;48(5):517-31. doi: 10.1111/cpr.12205. Epub 2015 Aug 13. Cell Prolif. 2015. PMID: 26269226 Free PMC article.
-
Orai3 and Orai1 mediate CRAC channel function and metabolic reprogramming in B cells.Elife. 2023 Feb 21;12:e84708. doi: 10.7554/eLife.84708. Elife. 2023. PMID: 36803766 Free PMC article.
-
Calcium Channels in Adult Brain Neural Stem Cells and in Glioblastoma Stem Cells.Front Cell Neurosci. 2020 Nov 13;14:600018. doi: 10.3389/fncel.2020.600018. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33281564 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
