Efficacy and safety of delayed-release dimethyl fumarate in patients newly diagnosed with relapsing-remitting multiple sclerosis (RRMS)
- PMID: 24990854
- PMCID: PMC4361464
- DOI: 10.1177/1352458514537013
Efficacy and safety of delayed-release dimethyl fumarate in patients newly diagnosed with relapsing-remitting multiple sclerosis (RRMS)
Abstract
Background: Delayed-release dimethyl fumarate (DMF) demonstrated efficacy and safety in the Phase 3 DEFINE and CONFIRM trials.
Objective: To evaluate delayed-release DMF in newly diagnosed relapsing-remitting multiple sclerosis (RRMS) patients, in a post-hoc analysis of integrated data from DEFINE and CONFIRM.
Methods: Patients included in the analysis were diagnosed with RRMS within 1 year prior to study entry and naive to MS disease-modifying therapy.
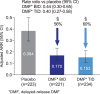
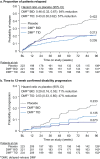
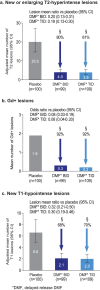
Results: The newly diagnosed population comprised 678 patients treated with placebo (n = 223) or delayed-release DMF 240 mg BID (n = 221) or TID (n = 234). At 2 years, delayed-release DMF BID and TID reduced the annualized relapse rate by 56% and 60% (both p < 0.0001), risk of relapse by 54% and 57% (both p < 0.0001), and risk of 12-week confirmed disability progression by 71% (p < 0.0001) and 47% (p = 0.0085) versus placebo. In a subset of patients (MRI cohort), delayed-release DMF BID and TID reduced the mean number of new or enlarging T2-hyperintense lesions by 80% and 81%, gadolinium-enhancing lesion activity by 92% and 92%, and mean number of new non-enhancing T1-hypointense lesions by 68% and 70% (all p < 0.0001 versus placebo). Flushing and gastrointestinal events were associated with delayed-release DMF.
Conclusion: Delayed-release DMF improved clinical and neuroradiological outcomes relative to placebo in newly diagnosed RRMS patients.
Keywords: Delayed-release dimethyl fumarate; efficacy; multiple sclerosis; newly diagnosed; safety.
© The Author(s), 2015.
Conflict of interest statement
Figures
Similar articles
-
Time course of clinical and neuroradiological effects of delayed-release dimethyl fumarate in multiple sclerosis.Eur J Neurol. 2015 Apr;22(4):664-71. doi: 10.1111/ene.12624. Epub 2015 Jan 2. Eur J Neurol. 2015. PMID: 25557371 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Delayed-release Dimethyl Fumarate for Relapsing-remitting Multiple Sclerosis in Prior Interferon Users: An Integrated Analysis of DEFINE and CONFIRM.Clin Ther. 2017 Aug;39(8):1671-1679. doi: 10.1016/j.clinthera.2017.06.012. Epub 2017 Jul 25. Clin Ther. 2017. PMID: 28751099 Clinical Trial.
-
Effects of delayed-release dimethyl fumarate on MRI measures in the Phase 3 DEFINE study.J Neurol. 2014 Sep;261(9):1794-802. doi: 10.1007/s00415-014-7412-x. Epub 2014 Jul 3. J Neurol. 2014. PMID: 24989666 Free PMC article. Clinical Trial.
-
[Extending therapeutic possibilities in relapsing-remitting multiple sclerosis: dimethyl fumarate].Ideggyogy Sz. 2015 Jan 30;68(1-2):7-14. Ideggyogy Sz. 2015. PMID: 25842911 Review. Hungarian.
-
Dimethyl fumarate for multiple sclerosis.Cochrane Database Syst Rev. 2015 Apr 22;2015(4):CD011076. doi: 10.1002/14651858.CD011076.pub2. Cochrane Database Syst Rev. 2015. PMID: 25900414 Free PMC article. Review.
Cited by
-
Long-Term Disability Outcomes in Relapsing-Remitting Multiple Sclerosis Patients: Impact of Clinical and Demographic Factors on Disease Progression.J Clin Med. 2024 Mar 21;13(6):1813. doi: 10.3390/jcm13061813. J Clin Med. 2024. PMID: 38542037 Free PMC article.
-
Updated Perspectives on the Challenges of Managing Multiple Sclerosis During Pregnancy.Degener Neurol Neuromuscul Dis. 2022 Jan 5;12:1-21. doi: 10.2147/DNND.S203406. eCollection 2022. Degener Neurol Neuromuscul Dis. 2022. PMID: 35023987 Free PMC article. Review.
-
Monomethyl Fumarate (MMF, Bafiertam) for the Treatment of Relapsing Forms of Multiple Sclerosis (MS).Neurol Int. 2021 May 19;13(2):207-223. doi: 10.3390/neurolint13020022. Neurol Int. 2021. PMID: 34069538 Free PMC article. Review.
-
Effectiveness of Dimethyl Fumarate in Patients With Relapsing Multiple Sclerosis Switching After Suboptimal Response to Glatiramer Acetate, Including Patients With Early Multiple Sclerosis: Subgroup Analysis of RESPOND.Neurol Ther. 2021 Jun;10(1):169-182. doi: 10.1007/s40120-020-00223-2. Epub 2020 Nov 23. Neurol Ther. 2021. PMID: 33225410 Free PMC article.
-
Advances in the management of relapsing-remitting multiple sclerosis: role of oral dimethyl fumarate (BG-12).Degener Neurol Neuromuscul Dis. 2015 May 21;5:51-61. doi: 10.2147/DNND.S68723. eCollection 2015. Degener Neurol Neuromuscul Dis. 2015. PMID: 32669912 Free PMC article. Review.
References
-
- Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372: 1502–1517. - PubMed
-
- Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998; 338: 278–285. - PubMed
-
- Comi G, Filippi M, Barkhof F, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet 2001; 357: 1576–1582. - PubMed
-
- Comi G, Martinelli V, Rodegher M, et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet 2009; 374: 1503–1511. - PubMed
-
- Comi G, De Stefano N, Freedman MS, et al. Comparison of two dosing frequencies of subcutaneous interferon beta-1a in patients with a first clinical demyelinating event suggestive of multiple sclerosis (REFLEX): a phase 3 randomised controlled trial. Lancet Neurol 2012; 11: 33–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

