Comparative validation of the D. melanogaster modENCODE transcriptome annotation
- PMID: 24985915
- PMCID: PMC4079975
- DOI: 10.1101/gr.159384.113
Comparative validation of the D. melanogaster modENCODE transcriptome annotation
Abstract
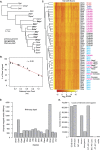
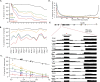
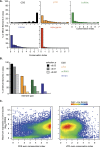
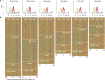
Accurate gene model annotation of reference genomes is critical for making them useful. The modENCODE project has improved the D. melanogaster genome annotation by using deep and diverse high-throughput data. Since transcriptional activity that has been evolutionarily conserved is likely to have an advantageous function, we have performed large-scale interspecific comparisons to increase confidence in predicted annotations. To support comparative genomics, we filled in divergence gaps in the Drosophila phylogeny by generating draft genomes for eight new species. For comparative transcriptome analysis, we generated mRNA expression profiles on 81 samples from multiple tissues and developmental stages of 15 Drosophila species, and we performed cap analysis of gene expression in D. melanogaster and D. pseudoobscura. We also describe conservation of four distinct core promoter structures composed of combinations of elements at three positions. Overall, each type of genomic feature shows a characteristic divergence rate relative to neutral models, highlighting the value of multispecies alignment in annotating a target genome that should prove useful in the annotation of other high priority genomes, especially human and other mammalian genomes that are rich in noncoding sequences. We report that the vast majority of elements in the annotation are evolutionarily conserved, indicating that the annotation will be an important springboard for functional genetic testing by the Drosophila community.
© 2014 Chen et al.; Published by Cold Spring Harbor Laboratory Press.
Figures

Similar articles
-
Correspondence of D. melanogaster and C. elegans developmental stages revealed by alternative splicing characteristics of conserved exons.BMC Genomics. 2017 Mar 16;18(1):234. doi: 10.1186/s12864-017-3600-2. BMC Genomics. 2017. PMID: 28302059 Free PMC article.
-
Deep sequencing the circadian and diurnal transcriptome of Drosophila brain.Genome Res. 2012 Jul;22(7):1266-81. doi: 10.1101/gr.128876.111. Epub 2012 Apr 3. Genome Res. 2012. PMID: 22472103 Free PMC article.
-
Assessing the impact of comparative genomic sequence data on the functional annotation of the Drosophila genome.Genome Biol. 2002;3(12):RESEARCH0086. doi: 10.1186/gb-2002-3-12-research0086. Epub 2002 Dec 30. Genome Biol. 2002. PMID: 12537575 Free PMC article.
-
Drosophila melanogaster: a case study of a model genomic sequence and its consequences.Genome Res. 2005 Dec;15(12):1661-7. doi: 10.1101/gr.3726705. Genome Res. 2005. PMID: 16339363 Review.
-
An Experimental Approach to Genome Annotation: This report is based on a colloquium sponsored by the American Academy of Microbiology held July 19-20, 2004, in Washington, DC.Washington (DC): American Society for Microbiology; 2004. Washington (DC): American Society for Microbiology; 2004. PMID: 33001599 Free Books & Documents. Review.
Cited by
-
Stochastic Modeling of Gene Expression Evolution Uncovers Tissue- and Sex-Specific Properties of Expression Evolution in the Drosophila Genus.J Comput Biol. 2023 Jan;30(1):21-40. doi: 10.1089/cmb.2022.0121. Epub 2022 Aug 26. J Comput Biol. 2023. PMID: 36037023 Free PMC article.
-
Unistrand piRNA clusters are an evolutionarily conserved mechanism to suppress endogenous retroviruses across the Drosophila genus.Nat Commun. 2023 Nov 13;14(1):7337. doi: 10.1038/s41467-023-42787-1. Nat Commun. 2023. PMID: 37957172 Free PMC article.
-
Drosophila kikkawai - Sox102F.MicroPubl Biol. 2024 Jul 19;2024:10.17912/micropub.biology.001211. doi: 10.17912/micropub.biology.001211. eCollection 2024. MicroPubl Biol. 2024. PMID: 39100195 Free PMC article.
-
Re-annotation of eight Drosophila genomes.Life Sci Alliance. 2018 Dec 24;1(6):e201800156. doi: 10.26508/lsa.201800156. eCollection 2018 Dec. Life Sci Alliance. 2018. PMID: 30599046 Free PMC article.
-
Deep Conservation of Hid-Like RHG Gene Family Homologs in Winged Insects Revealed by "Taxon Hopping" BLAST.Insects. 2021 Oct 21;12(11):957. doi: 10.3390/insects12110957. Insects. 2021. PMID: 34821758 Free PMC article.
References
-
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. 2000. The genome sequence of Drosophila melanogaster. Science 287: 2185–2195 - PubMed
-
- Balakirev ES, Ayala FJ 2003. Pseudogenes: are they “junk” or functional DNA? Annu Rev Genet 37: 123–151 - PubMed
-
- Banerji J, Olson L, Schaffner W 1983. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell 33: 729–740 - PubMed
-
- Bass B, Hundley H, Li JB, Peng Z, Pickrell J, Xiao XG, Yang L 2012. The difficult calls in RNA editing. Nat Biotechnol 30: 1207–1209 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
