Branched-linear and agglomerate protein polymers as vaccine platforms
- PMID: 24985736
- PMCID: PMC4137571
- DOI: 10.1016/j.biomaterials.2014.06.021
Branched-linear and agglomerate protein polymers as vaccine platforms
Abstract
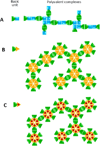
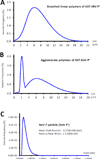
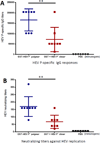
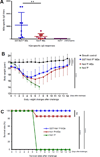
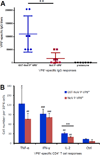
Many viral structural proteins and their truncated domains share a common feature of homotypic interaction forming dimers, trimers, and/or oligomers with various valences. We reported previously a simple strategy for construction of linear and network polymers through the dimerization feature of viral proteins for vaccine development. In this study, technologies were developed to produce more sophisticated polyvalent complexes through both the dimerization and oligomerization natures of viral antigens. As proof of concept, branched-linear and agglomerate polymers were made via fusions of the dimeric glutathione-s-transferase (GST) with either a tetrameric hepatitis E virus (HEV) protruding protein or a 24-meric norovirus (NoV) protruding protein. Furthermore, a monomeric antigen, either the M2e epitope of influenza A virus or the VP8* antigen of rotavirus, was inserted and displayed by the polymer platform. All resulting polymers were easily produced in Escherichia coli at high yields. Immunization of mice showed that the polymer vaccines induced significantly higher specific humoral and T cell responses than those induced by the dimeric antigens. Additional evidence in supporting use of polymer vaccines included the significantly higher neutralization activity and protective immunity of the polymer vaccines against the corresponding viruses than those of the dimer vaccines. Thus, our technology for production of polymers containing different viral antigens offers a strategy for vaccine development against infectious pathogens and their associated diseases.
Keywords: Flu vaccine; Hepatitis E virus vaccine; Norovirus; Norovirus vaccine; Vaccine development; Vaccine platform.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
A trivalent vaccine candidate against hepatitis E virus, norovirus, and astrovirus.Vaccine. 2016 Feb 10;34(7):905-13. doi: 10.1016/j.vaccine.2015.12.068. Epub 2016 Jan 9. Vaccine. 2016. PMID: 26778421 Free PMC article.
-
A dual vaccine candidate against norovirus and hepatitis E virus.Vaccine. 2014 Jan 16;32(4):445-52. doi: 10.1016/j.vaccine.2013.11.064. Epub 2013 Nov 27. Vaccine. 2014. PMID: 24291540 Free PMC article.
-
Polyvalent complexes for vaccine development.Biomaterials. 2013 Jun;34(18):4480-92. doi: 10.1016/j.biomaterials.2013.02.041. Epub 2013 Mar 15. Biomaterials. 2013. PMID: 23498893 Free PMC article.
-
Norovirus P particle: a subviral nanoparticle for vaccine development against norovirus, rotavirus and influenza virus.Nanomedicine (Lond). 2012 Jun;7(6):889-97. doi: 10.2217/nnm.12.62. Nanomedicine (Lond). 2012. PMID: 22734641 Free PMC article. Review.
-
Vaccine against norovirus.Hum Vaccin Immunother. 2014;10(6):1449-56. doi: 10.4161/hv.28626. Epub 2014 May 5. Hum Vaccin Immunother. 2014. PMID: 24718366 Free PMC article. Review.
Cited by
-
Recent advancements in combination subunit vaccine development.Hum Vaccin Immunother. 2017 Jan 2;13(1):180-185. doi: 10.1080/21645515.2016.1229719. Hum Vaccin Immunother. 2017. PMID: 27649319 Free PMC article.
-
Saliva as a source of reagent to study human susceptibility to avian influenza H7N9 virus infection.Emerg Microbes Infect. 2018 Sep 19;7(1):156. doi: 10.1038/s41426-018-0160-8. Emerg Microbes Infect. 2018. PMID: 30228261 Free PMC article.
-
Affinities of human histo-blood group antigens for norovirus capsid protein complexes.Glycobiology. 2015 Feb;25(2):170-80. doi: 10.1093/glycob/cwu100. Epub 2014 Oct 1. Glycobiology. 2015. PMID: 25395406 Free PMC article.
-
A trivalent vaccine candidate against hepatitis E virus, norovirus, and astrovirus.Vaccine. 2016 Feb 10;34(7):905-13. doi: 10.1016/j.vaccine.2015.12.068. Epub 2016 Jan 9. Vaccine. 2016. PMID: 26778421 Free PMC article.
-
Gangliosides are ligands for human noroviruses.J Am Chem Soc. 2014 Sep 10;136(36):12631-7. doi: 10.1021/ja505272n. Epub 2014 Aug 28. J Am Chem Soc. 2014. PMID: 25105447 Free PMC article.
References
-
- McAleer WJ, Buynak EB, Maigetter RZ, Wampler DE, Miller WJ, Hilleman MR. Human hepatitis B vaccine from recombinant yeast. Nature. 1984;307:178–180. - PubMed
-
- Andre FE, Safary A. Summary of clinical findings on Engerix-B, a genetically engineered yeast derived hepatitis B vaccine. Postgrad Med J. 1987;63(Suppl 2):169–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

