Reciprocal regulation of endocytosis and metabolism
- PMID: 24984778
- PMCID: PMC4067987
- DOI: 10.1101/cshperspect.a016964
Reciprocal regulation of endocytosis and metabolism
Abstract
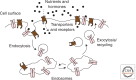
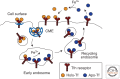
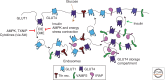
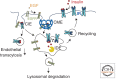
The cellular uptake of many nutrients and micronutrients governs both their cellular availability and their systemic homeostasis. The cellular rate of nutrient or ion uptake (e.g., glucose, Fe(3+), K(+)) or efflux (e.g., Na(+)) is governed by a complement of membrane transporters and receptors that show dynamic localization at both the plasma membrane and defined intracellular membrane compartments. Regulation of the rate and mechanism of endocytosis controls the amounts of these proteins on the cell surface, which in many cases determines nutrient uptake or secretion. Moreover, the metabolic action of diverse hormones is initiated upon binding to surface receptors that then undergo regulated endocytosis and show distinct signaling patterns once internalized. Here, we examine how the endocytosis of nutrient transporters and carriers as well as signaling receptors governs cellular metabolism and thereby systemic (whole-body) metabolite homeostasis.
Copyright © 2014 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
Similar articles
-
TRP Channel Trafficking.In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. PMID: 21204515 Free Books & Documents. Review.
-
The GLUT4 glucose transporter.Cell Metab. 2007 Apr;5(4):237-52. doi: 10.1016/j.cmet.2007.03.006. Cell Metab. 2007. PMID: 17403369 Review.
-
Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160.Mol Biol Cell. 2004 Oct;15(10):4406-15. doi: 10.1091/mbc.e04-04-0333. Epub 2004 Jul 14. Mol Biol Cell. 2004. PMID: 15254270 Free PMC article.
-
Documenting GLUT4 exocytosis and endocytosis in muscle cell monolayers.Curr Protoc Cell Biol. 2010 Mar;Chapter 15:Unit 15.15. doi: 10.1002/0471143030.cb1515s46. Curr Protoc Cell Biol. 2010. PMID: 20235101
-
Intracellular insulin-responsive glucose transporter (GLUT4) distribution but not insulin-stimulated GLUT4 exocytosis and recycling are microtubule dependent.Mol Endocrinol. 2002 May;16(5):1060-8. doi: 10.1210/mend.16.5.0836. Mol Endocrinol. 2002. PMID: 11981040
Cited by
-
AMP-Activated Protein Kinase Regulates the Cell Surface Proteome and Integrin Membrane Traffic.PLoS One. 2015 May 26;10(5):e0128013. doi: 10.1371/journal.pone.0128013. eCollection 2015. PLoS One. 2015. PMID: 26010094 Free PMC article.
-
Endosome-mitochondria interactions are modulated by iron release from transferrin.J Cell Biol. 2016 Sep 26;214(7):831-45. doi: 10.1083/jcb.201602069. Epub 2016 Sep 19. J Cell Biol. 2016. PMID: 27646275 Free PMC article.
-
Inducible Exoc7/Exo70 knockout reveals a critical role of the exocyst in insulin-regulated GLUT4 exocytosis.J Biol Chem. 2019 Dec 27;294(52):19988-19996. doi: 10.1074/jbc.RA119.010821. Epub 2019 Nov 18. J Biol Chem. 2019. PMID: 31740584 Free PMC article.
-
Selective regulation of clathrin-mediated epidermal growth factor receptor signaling and endocytosis by phospholipase C and calcium.Mol Biol Cell. 2017 Oct 15;28(21):2802-2818. doi: 10.1091/mbc.E16-12-0871. Epub 2017 Aug 16. Mol Biol Cell. 2017. PMID: 28814502 Free PMC article.
-
Genetic evidence for an inhibitory role of tomosyn in insulin-stimulated GLUT4 exocytosis.Traffic. 2020 Oct;21(10):636-646. doi: 10.1111/tra.12760. Traffic. 2020. PMID: 32851733 Free PMC article.
References
-
- Acconcia F, Sigismund S, Polo S 2009. Ubiquitin in trafficking: The network at work. Exp Cell Res 315: 1610–1618 - PubMed
-
- Antonescu CN, Diaz M, Femia G, Planas JV, Klip A 2008a. Clathrin-dependent and independent endocytosis of glucose transporter 4 (GLUT4) in myoblasts: Regulation by mitochondrial uncoupling. Traffic 9: 1173–1190 - PubMed
-
- Antonescu CN, Randhawa VK, Klip A 2008b. Dissecting GLUT4 traffic components in L6 myocytes by fluorescence-based, single-cell assays. Meth Mol Biol 457: 367–378 - PubMed
-
- Antonescu CN, Foti M, Sauvonnet N, Klip A 2009. Ready, set, internalize: Mechanisms and regulation of GLUT4 endocytosis. Biosci Rep 29: 1–11 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
