Lipoteichoic acid (LTA) and lipopolysaccharides (LPS) from periodontal pathogenic bacteria facilitate oncogenic herpesvirus infection within primary oral cells
- PMID: 24971655
- PMCID: PMC4074159
- DOI: 10.1371/journal.pone.0101326
Lipoteichoic acid (LTA) and lipopolysaccharides (LPS) from periodontal pathogenic bacteria facilitate oncogenic herpesvirus infection within primary oral cells
Abstract
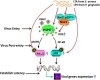
Kaposi's sarcoma (KS) remains the most common tumor arising in patients with HIV/AIDS, and involvement of the oral cavity represents one of the most common clinical manifestations of this tumor. HIV infection incurs an increased risk for periodontal diseases and oral carriage of a variety of bacteria. Whether interactions involving pathogenic bacteria and oncogenic viruses in the local environment facilitate replication or maintenance of these viruses in the oral cavity remains unknown. In the current study, our data indicate that pretreatment of primary human oral fibroblasts with two prototypical pathogen-associated molecular patterns (PAMPs) produced by oral pathogenic bacteria-lipoteichoic acid (LTA) and lipopolysaccharide (LPS), increase KSHV entry and subsequent viral latent gene expression during de novo infection. Further experiments demonstrate that the underlying mechanisms induced by LTA and/or LPS include upregulation of cellular receptor, increasing production of reactive oxygen species (ROS), and activating intracellular signaling pathways such as MAPK and NF-κB, and all of which are closely associated with KSHV entry or gene expression within oral cells. Based on these findings, we hope to provide the framework of developing novel targeted approaches for treatment and prevention of oral KSHV infection and KS development in high-risk HIV-positive patients.
Conflict of interest statement
Figures

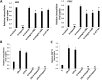

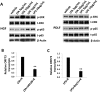
 = p<0.01 relative to K (**), LTA+K (##), and LPS+K (
= p<0.01 relative to K (**), LTA+K (##), and LPS+K (
 ).
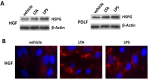
).
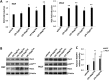
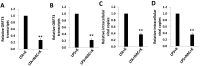
Similar articles
-
Kaposi Sarcoma-Associated Herpesvirus and Staphylococcus aureus Coinfection in Oral Cavities of HIV-Positive Patients: A Unique Niche for Oncogenic Virus Lytic Reactivation.J Infect Dis. 2020 Mar 28;221(8):1331-1341. doi: 10.1093/infdis/jiz249. J Infect Dis. 2020. PMID: 31111897 Free PMC article.
-
Porphyromonas gingivalis coinfects with KSHV in oral cavities of HIV+ patients and induces viral lytic reactivation.J Med Virol. 2020 Dec;92(12):3862-3867. doi: 10.1002/jmv.26028. Epub 2020 Jun 2. J Med Virol. 2020. PMID: 32436999 Free PMC article.
-
Pseudomonas aeruginosa Stimulates Inflammation and Enhances Kaposi's Sarcoma Herpesvirus-Induced Cell Proliferation and Cellular Transformation through both Lipopolysaccharide and Flagellin.mBio. 2020 Nov 10;11(6):e02843-20. doi: 10.1128/mBio.02843-20. mBio. 2020. PMID: 33173008 Free PMC article.
-
Clinical Manifestations and Epigenetic Regulation of Oral Herpesvirus Infections.Viruses. 2021 Apr 15;13(4):681. doi: 10.3390/v13040681. Viruses. 2021. PMID: 33920978 Free PMC article. Review.
-
Cyclooxygenase-2-prostaglandin E2-eicosanoid receptor inflammatory axis: a key player in Kaposi's sarcoma-associated herpes virus associated malignancies.Transl Res. 2013 Aug;162(2):77-92. doi: 10.1016/j.trsl.2013.03.004. Epub 2013 Apr 6. Transl Res. 2013. PMID: 23567332 Free PMC article. Review.
Cited by
-
Virus-Bacteria Interactions: An Emerging Topic in Human Infection.Viruses. 2017 Mar 21;9(3):58. doi: 10.3390/v9030058. Viruses. 2017. PMID: 28335562 Free PMC article. Review.
-
Bacterial-Viral Interactions in Human Orodigestive and Female Genital Tract Cancers: A Summary of Epidemiologic and Laboratory Evidence.Cancers (Basel). 2022 Jan 15;14(2):425. doi: 10.3390/cancers14020425. Cancers (Basel). 2022. PMID: 35053587 Free PMC article. Review.
-
xCT, not just an amino-acid transporter: a multi-functional regulator of microbial infection and associated diseases.Front Microbiol. 2015 Feb 19;6:120. doi: 10.3389/fmicb.2015.00120. eCollection 2015. Front Microbiol. 2015. PMID: 25745420 Free PMC article.
-
Co-infections and Pathogenesis of KSHV-Associated Malignancies.Front Microbiol. 2016 Feb 15;7:151. doi: 10.3389/fmicb.2016.00151. eCollection 2016. Front Microbiol. 2016. PMID: 26913028 Free PMC article. Review.
-
High Glucose Induces Reactivation of Latent Kaposi's Sarcoma-Associated Herpesvirus.J Virol. 2016 Oct 14;90(21):9654-9663. doi: 10.1128/JVI.01049-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27535045 Free PMC article.
References
-
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, et al. (1994) Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 266: 1865–1869. - PubMed
-
- Bonnet F, Lewden C, May T, Heripret L, Jougla E, et al. (2004) Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer 101: 317–324. - PubMed
-
- Lebbe C, Legendre C, Frances C (2008) Kaposi sarcoma in transplantation. Transplant Rev (Orlando) 22: 252–261. - PubMed
-
- Vanni T, Sprinz E, Machado MW, Santana Rde C, Fonseca BA, et al. (2006) Systemic treatment of AIDS-related Kaposi sarcoma: current status and perspectives. Cancer Treat Rev 32: 445–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

