Monocytes are recruited from venules during arteriogenesis in the murine spinotrapezius ligation model
- PMID: 24969773
- PMCID: PMC4373588
- DOI: 10.1161/ATVBAHA.114.303399
Monocytes are recruited from venules during arteriogenesis in the murine spinotrapezius ligation model
Abstract
Objective: Chronic arterial occlusion results in arteriogenesis of collateral blood vessels. This process has been shown to be dependent on the recruitment of growth-promoting macrophages to remodeling collaterals. However, the potential role of venules in monocyte recruitment during microvascular arteriogenesis is not well demonstrated. First, we aim to document that arteriogenesis occurs in the mouse spinotrapezius ligation model. Then, we investigate the temporal and spatial distribution, as well as proliferation, of monocytes/macrophages recruited to collateral arterioles in response to elevated fluid shear stress.
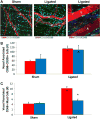
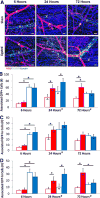
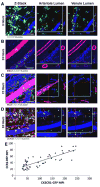
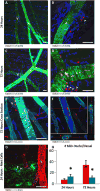
Approach and results: Laser speckle flowmetry confirmed a postligation increase in blood velocity within collateral arterioles but not within venules. After 72 hours post ligation, collateral arteriole diameters were increased, proliferating cells were identified in vessel walls of shear-activated collaterals, and perivascular CD206(+) macrophages demonstrated proliferation. A 5-ethynyl-2'-deoxyuridine assay identified proliferation. CD68(+)CD206(+) cells around collaterals were increased 96%, whereas CX3CR1((+/GFP)) cells were increased 126% in ligated versus sham groups after 72 hours. CX3CR1((+/GFP)) cells were predominately venule associated at 6 hours after ligation; and CX3CR1((+/GFP hi)) cells shifted from venule to arteriole associated between 6 and 72 hours after surgery exclusively in ligated muscle. We report accumulation and extravasation of adhered CX3CR1((+/GFP)) cells in and from venules, but not from arterioles, after ligation.
Conclusions: Our results demonstrate that arteriogenesis occurs in the murine spinotrapezius ligation model and implicate postcapillary venules as the site of tissue entry for circulating monocytes. Local proliferation of macrophages is also documented. These data open up questions about the role of arteriole-venule communication during monocyte recruitment.
Keywords: arterioles; blood flow velocity; macrophages; microvasculature; monocytes.
© 2014 American Heart Association, Inc.
Figures


Similar articles
-
P2Y2 nucleotide receptor mediates arteriogenesis in a murine model of hind limb ischemia.J Vasc Surg. 2016 Jan;63(1):216-25. doi: 10.1016/j.jvs.2014.06.112. Epub 2014 Jul 31. J Vasc Surg. 2016. PMID: 25088742 Free PMC article.
-
Arteriogenesis in murine adipose tissue is contingent on CD68+ /CD206+ macrophages.Microcirculation. 2017 May;24(4):10.1111/micc.12341. doi: 10.1111/micc.12341. Microcirculation. 2017. PMID: 27976451 Free PMC article.
-
Protease-activated receptor (PAR)2, but not PAR1, is involved in collateral formation and anti-inflammatory monocyte polarization in a mouse hind limb ischemia model.PLoS One. 2013 Apr 18;8(4):e61923. doi: 10.1371/journal.pone.0061923. Print 2013. PLoS One. 2013. PMID: 23637930 Free PMC article.
-
The chemokine system in arteriogenesis and hind limb ischemia.J Vasc Surg. 2007 Jun;45 Suppl A(Suppl A):A48-56. doi: 10.1016/j.jvs.2007.02.030. J Vasc Surg. 2007. PMID: 17544024 Free PMC article. Review.
-
Collateral circulation: past and present.Basic Res Cardiol. 2009 Jan;104(1):5-21. doi: 10.1007/s00395-008-0760-x. Epub 2008 Dec 20. Basic Res Cardiol. 2009. PMID: 19101749 Free PMC article. Review.
Cited by
-
Vascular growth responses to chronic arterial occlusion are unaffected by myeloid specific focal adhesion kinase (FAK) deletion.Sci Rep. 2016 May 31;6:27029. doi: 10.1038/srep27029. Sci Rep. 2016. PMID: 27244251 Free PMC article.
-
NFAT5 promotes arteriogenesis via MCP-1-dependent monocyte recruitment.J Cell Mol Med. 2020 Jan;24(2):2052-2063. doi: 10.1111/jcmm.14904. Epub 2019 Dec 28. J Cell Mol Med. 2020. PMID: 31883300 Free PMC article.
-
Exposure of Endothelium to Biomimetic Flow Waveforms Yields Identification of miR-199a-5p as a Potent Regulator of Arteriogenesis.Mol Ther Nucleic Acids. 2018 Sep 7;12:829-844. doi: 10.1016/j.omtn.2018.08.001. Epub 2018 Aug 8. Mol Ther Nucleic Acids. 2018. PMID: 30153567 Free PMC article.
-
Selective recruitment of non-classical monocytes promotes skeletal muscle repair.Biomaterials. 2017 Feb;117:32-43. doi: 10.1016/j.biomaterials.2016.11.021. Epub 2016 Nov 16. Biomaterials. 2017. PMID: 27930948 Free PMC article.
-
Macrophages: An Inflammatory Link Between Angiogenesis and Lymphangiogenesis.Microcirculation. 2016 Feb;23(2):95-121. doi: 10.1111/micc.12259. Microcirculation. 2016. PMID: 26614117 Free PMC article. Review.
References
-
- Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004 Feb 6;94:230–238. - PubMed
-
- Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol. 2002 Dec;283:H2411–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

