PKA-regulated VASP phosphorylation promotes extrusion of transformed cells from the epithelium
- PMID: 24963131
- PMCID: PMC4132389
- DOI: 10.1242/jcs.149674
PKA-regulated VASP phosphorylation promotes extrusion of transformed cells from the epithelium
Abstract
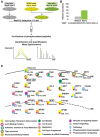
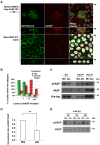
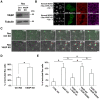
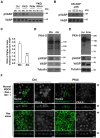
At the early stages of carcinogenesis, transformation occurs in single cells within tissues. In an epithelial monolayer, such mutated cells are recognized by their normal neighbors and are often apically extruded. The apical extrusion requires cytoskeletal reorganization and changes in cell shape, but the molecular switches involved in the regulation of these processes are poorly understood. Here, using stable isotope labeling by amino acids in cell culture (SILAC)-based quantitative mass spectrometry, we have identified proteins that are modulated in transformed cells upon their interaction with normal cells. Phosphorylation of VASP at serine 239 is specifically upregulated in Ras(V12)-transformed cells when they are surrounded by normal cells. VASP phosphorylation is required for the cell shape changes and apical extrusion of Ras-transformed cells. Furthermore, PKA is activated in Ras-transformed cells that are surrounded by normal cells, leading to VASP phosphorylation. These results indicate that the PKA-VASP pathway is a crucial regulator of tumor cell extrusion from the epithelium, and they shed light on the events occurring at the early stage of carcinogenesis.
Keywords: Apical extrusion; PKA; Ras-transformed; VASP.
© 2014. Published by The Company of Biologists Ltd.
Figures
Similar articles
-
EPLIN is a crucial regulator for extrusion of RasV12-transformed cells.J Cell Sci. 2015 Feb 15;128(4):781-9. doi: 10.1242/jcs.163113. Epub 2015 Jan 20. J Cell Sci. 2015. PMID: 25609711
-
Regulation of vasodilator-stimulated phosphoprotein phosphorylation and interaction with Abl by protein kinase A and cell adhesion.J Biol Chem. 2002 Oct 11;277(41):38121-6. doi: 10.1074/jbc.M205379200. Epub 2002 Jun 26. J Biol Chem. 2002. PMID: 12087107
-
Promotion of PDGF-induced endothelial cell migration by phosphorylated VASP depends on PKA anchoring via AKAP.Mol Cell Biochem. 2010 Feb;335(1-2):1-11. doi: 10.1007/s11010-009-0234-y. Epub 2009 Aug 27. Mol Cell Biochem. 2010. PMID: 19711176
-
Interface between normal and transformed epithelial cells: a road to a novel type of cancer prevention and treatment.Cancer Sci. 2011 Oct;102(10):1749-55. doi: 10.1111/j.1349-7006.2011.02011.x. Epub 2011 Jul 21. Cancer Sci. 2011. PMID: 21692919 Free PMC article. Review.
-
Early mechanical selection of cell extrusion and extrusion signaling in cancer.Curr Opin Cell Biol. 2021 Oct;72:36-40. doi: 10.1016/j.ceb.2021.04.005. Epub 2021 May 24. Curr Opin Cell Biol. 2021. PMID: 34034216 Free PMC article. Review.
Cited by
-
Pleiotropic effects of cell competition between normal and transformed cells in mammalian cancers.J Cancer Res Clin Oncol. 2023 Apr;149(4):1607-1619. doi: 10.1007/s00432-022-04143-6. Epub 2022 Jul 7. J Cancer Res Clin Oncol. 2023. PMID: 35796779 Free PMC article. Review.
-
Src-transformed cells hijack mitosis to extrude from the epithelium.Nat Commun. 2018 Nov 8;9(1):4695. doi: 10.1038/s41467-018-07163-4. Nat Commun. 2018. PMID: 30410020 Free PMC article.
-
Changes in Vasodilator-Stimulated Phosphoprotein Phosphorylation, Profilin-1, and Cofilin-1 in Accreta and Protection by DHA.Reprod Sci. 2019 Jun;26(6):757-765. doi: 10.1177/1933719118792095. Epub 2018 Aug 9. Reprod Sci. 2019. PMID: 30092744 Free PMC article.
-
Desmosomes polarize and integrate chemical and mechanical signaling to govern epidermal tissue form and function.Curr Biol. 2021 Aug 9;31(15):3275-3291.e5. doi: 10.1016/j.cub.2021.05.021. Epub 2021 Jun 8. Curr Biol. 2021. PMID: 34107301 Free PMC article.
-
Alterative Expression and Localization of Profilin 1/VASPpS157 and Cofilin 1/VASPpS239 Regulates Metastatic Growth and Is Modified by DHA Supplementation.Mol Cancer Ther. 2016 Sep;15(9):2220-31. doi: 10.1158/1535-7163.MCT-16-0092. Epub 2016 Aug 5. Mol Cancer Ther. 2016. PMID: 27496138 Free PMC article.
References
-
- Bear J. E., Svitkina T. M., Krause M., Schafer D. A., Loureiro J. J., Strasser G. A., Maly I. V., Chaga O. Y., Cooper J. A., Borisy G. G. et al.(2002). Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 109, 509–521 10.1016/S0092-8674(02)00731-6 - DOI - PubMed
-
- Becker E. M., Schmidt P., Schramm M., Schröder H., Walter U., Hoenicka M., Gerzer R., Stasch J. P. (2000). The vasodilator-stimulated phosphoprotein (VASP): target of YC-1 and nitric oxide effects in human and rat platelets. J. Cardiovasc. Pharmacol. 35, 390–397 10.1097/00005344-200003000-00007 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

