Mucosal polyinosinic-polycytidylic acid improves protection elicited by replicating influenza vaccines via enhanced dendritic cell function and T cell immunity
- PMID: 24958904
- PMCID: PMC4111144
- DOI: 10.4049/jimmunol.1400222
Mucosal polyinosinic-polycytidylic acid improves protection elicited by replicating influenza vaccines via enhanced dendritic cell function and T cell immunity
Abstract
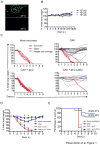
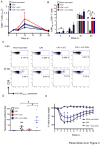
Live-attenuated influenza vaccines (LAIVs) have the potential to generate CD8 T cell immunity that may limit the virulence of an antigenically shifted influenza strain in a population lacking protective Abs. However, current LAIVs exert limited T cell immunity restricted to the vaccine strains. One approach to improve LAIV-induced T cell responses is the use of specific adjuvants to enhance T cell priming by respiratory dendritic cells, but this hypothesis has not been addressed. In this study, we assessed the effect of the TLR3 ligand polyinosinic-polycytidylic acid (poly IC) on CD8 T cell immunity and protection elicited by LAIVs. Mucosal treatment with poly IC shortly after vaccination enhanced respiratory dendritic cell function, CD8 T cell formation, and production of neutralizing Abs. This adjuvant effect of poly IC was dependent on amplification of TLR3 signaling by nonhematopoietic radioresistant cells and enhanced mouse protection to homosubtypic, as well as heterosubtypic, virus challenge. Our findings indicate that mucosal TLR3 ligation may be used to improve CD8 T cell responses to replicating vaccines, which has implications for protection in the absence of pre-existing Ab immunity.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures
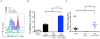
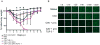
Similar articles
-
Establishment of memory CD8+ T cells with live attenuated influenza virus across different vaccination doses.J Gen Virol. 2016 Dec;97(12):3205-3214. doi: 10.1099/jgv.0.000651. Epub 2016 Nov 2. J Gen Virol. 2016. PMID: 27902386
-
Generation of DelNS1 Influenza Viruses: a Strategy for Optimizing Live Attenuated Influenza Vaccines.mBio. 2019 Sep 17;10(5):e02180-19. doi: 10.1128/mBio.02180-19. mBio. 2019. PMID: 31530680 Free PMC article.
-
An alphavirus-based adjuvant enhances serum and mucosal antibodies, T cells, and protective immunity to influenza virus in neonatal mice.J Virol. 2014 Aug;88(16):9182-96. doi: 10.1128/JVI.00327-14. Epub 2014 Jun 4. J Virol. 2014. PMID: 24899195 Free PMC article.
-
Novel G3/DT adjuvant promotes the induction of protective T cells responses after vaccination with a seasonal trivalent inactivated split-virion influenza vaccine.Vaccine. 2014 Sep 29;32(43):5614-23. doi: 10.1016/j.vaccine.2014.08.003. Epub 2014 Aug 17. Vaccine. 2014. PMID: 25140929
-
Insights into current clinical research on the immunogenicity of live attenuated influenza vaccines.Expert Rev Vaccines. 2020 Jan;19(1):43-55. doi: 10.1080/14760584.2020.1711056. Epub 2020 Jan 18. Expert Rev Vaccines. 2020. PMID: 31903816 Review.
Cited by
-
Antibodies to the Glycoprotein GP2 Subunit Cross-React between Old and New World Arenaviruses.mSphere. 2018 May 2;3(3):e00189-18. doi: 10.1128/mSphere.00189-18. eCollection 2018 May-Jun. mSphere. 2018. PMID: 29720525 Free PMC article.
-
Prospects of HA-based universal influenza vaccine.Biomed Res Int. 2015;2015:414637. doi: 10.1155/2015/414637. Epub 2015 Feb 15. Biomed Res Int. 2015. PMID: 25785268 Free PMC article. Review.
-
Protective efficacy of intranasal inactivated pseudorabies vaccine is improved by combination adjuvant in mice.Front Microbiol. 2022 Sep 15;13:976220. doi: 10.3389/fmicb.2022.976220. eCollection 2022. Front Microbiol. 2022. PMID: 36187997 Free PMC article.
-
Intranasal Administration of Recombinant Influenza Vaccines in Chimeric Mouse Models to Study Mucosal Immunity.J Vis Exp. 2015 Jun 25;(100):e52803. doi: 10.3791/52803. J Vis Exp. 2015. PMID: 26168339 Free PMC article.
-
A Novel Bacterium-Like Particle-Based Vaccine Displaying the SUDV Glycoprotein Induces Potent Humoral and Cellular Immune Responses in Mice.Viruses. 2019 Dec 11;11(12):1149. doi: 10.3390/v11121149. Viruses. 2019. PMID: 31835785 Free PMC article.
References
-
- Thomas PG, Brown SA, Keating R, Yue W, Morris MY, So J, Webby RJ, Doherty PC. Hidden epitopes emerge in secondary influenza virus-specific CD8+ T cell responses. J Immunol. 2007;178:3091–3098. - PubMed
-
- Epstein SL, Kong WP, Misplon JA, Lo CY, Tumpey TM, Xu L, Nabel GJ. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine. 2005;23:5404–5410. - PubMed
-
- Nabel GJ, Fauci AS. Induction of unnatural immunity: prospects for a broadly protective universal influenza vaccine. Nat Med. 2010;16:1389–1391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

