A small-molecule inhibitor of PIM kinases as a potential treatment for urothelial carcinomas
- PMID: 24953177
- PMCID: PMC4198696
- DOI: 10.1016/j.neo.2014.05.004
A small-molecule inhibitor of PIM kinases as a potential treatment for urothelial carcinomas
Abstract
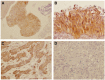
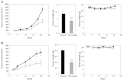
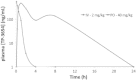
The proto-oncogene proviral integration site for moloney murine leukemia virus (PIM) kinases (PIM-1, PIM-2, and PIM-3) are serine/threonine kinases that are involved in a number of signaling pathways important to cancer cells. PIM kinases act in downstream effector functions as inhibitors of apoptosis and as positive regulators of G1-S phase progression through the cell cycle. PIM kinases are upregulated in multiple cancer indications, including lymphoma, leukemia, multiple myeloma, and prostate, gastric, and head and neck cancers. Overexpression of one or more PIM family members in patient tumors frequently correlates with poor prognosis. The aim of this investigation was to evaluate PIM expression in low- and high-grade urothelial carcinoma and to assess the role PIM function in disease progression and their potential to serve as molecular targets for therapy. One hundred thirty-seven cases of urothelial carcinoma were included in this study of surgical biopsy and resection specimens. High levels of expression of all three PIM family members were observed in both noninvasive and invasive urothelial carcinomas. The second-generation PIM inhibitor, TP-3654, displays submicromolar activity in pharmacodynamic biomarker modulation, cell proliferation studies, and colony formation assays using the UM-UC-3 bladder cancer cell line. TP-3654 displays favorable human ether-à-go-go-related gene and cytochrome P450 inhibition profiles compared with the first-generation PIM inhibitor, SGI-1776, and exhibits oral bioavailability. In vivo xenograft studies using a bladder cancer cell line show that PIM kinase inhibition can reduce tumor growth, suggesting that PIM kinase inhibitors may be active in human urothelial carcinomas.
Copyright © 2014. Published by Elsevier Inc.
Figures



Similar articles
-
Transcription and translation are primary targets of Pim kinase inhibitor SGI-1776 in mantle cell lymphoma.Blood. 2012 Oct 25;120(17):3491-500. doi: 10.1182/blood-2012-02-412643. Epub 2012 Sep 6. Blood. 2012. PMID: 22955922 Free PMC article.
-
The Pim kinases: new targets for drug development.Curr Drug Targets. 2011 Dec;12(14):2059-66. doi: 10.2174/138945011798829447. Curr Drug Targets. 2011. PMID: 21777193 Review.
-
Anti-tumour effects of PIM kinase inhibition on progression and chemoresistance of hepatocellular carcinoma.J Pathol. 2020 Sep;252(1):65-76. doi: 10.1002/path.5492. Epub 2020 Jul 31. J Pathol. 2020. PMID: 32558942
-
Targeting PIM kinase enhances the activity of sunitinib in renal cell carcinoma.Br J Cancer. 2011 Nov 8;105(10):1563-73. doi: 10.1038/bjc.2011.426. Epub 2011 Oct 20. Br J Cancer. 2011. PMID: 22015557 Free PMC article.
-
A review on structure-function mechanism and signaling pathway of serine/threonine protein PIM kinases as a therapeutic target.Int J Biol Macromol. 2024 Jun;270(Pt 1):132030. doi: 10.1016/j.ijbiomac.2024.132030. Epub 2024 May 3. Int J Biol Macromol. 2024. PMID: 38704069 Review.
Cited by
-
Design, synthesis, and anti-breast cancer activity evaluation of novel 3-cyanopyridine derivatives as PIM-1 inhibitors.Mol Divers. 2024 Nov 9. doi: 10.1007/s11030-024-11010-8. Online ahead of print. Mol Divers. 2024. PMID: 39521748
-
A novel, dual pan-PIM/FLT3 inhibitor SEL24 exhibits broad therapeutic potential in acute myeloid leukemia.Oncotarget. 2018 Mar 30;9(24):16917-16931. doi: 10.18632/oncotarget.24747. eCollection 2018 Mar 30. Oncotarget. 2018. PMID: 29682194 Free PMC article.
-
PIM1 kinase as a promise of targeted therapy in prostate cancer stem cells.Mol Clin Oncol. 2016 Jan;4(1):13-17. doi: 10.3892/mco.2015.673. Epub 2015 Nov 9. Mol Clin Oncol. 2016. PMID: 26835011 Free PMC article.
-
Targeted therapies in bladder cancer: an overview of in vivo research.Nat Rev Urol. 2015 Dec;12(12):681-94. doi: 10.1038/nrurol.2015.231. Epub 2015 Sep 22. Nat Rev Urol. 2015. PMID: 26390971 Review.
-
Synthesis and Biological Evaluation of Pyrazolo[1,5-a]pyrimidine Compounds as Potent and Selective Pim-1 Inhibitors.ACS Med Chem Lett. 2014 Oct 22;6(1):63-7. doi: 10.1021/ml500300c. eCollection 2015 Jan 8. ACS Med Chem Lett. 2014. PMID: 25589932 Free PMC article.
References
-
- Cuypers HT, Selten G, Quint W, Zijlstra M, Maandag ER, Boelens W, van Wezenbeek P, Melief C, Berns A. Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region. Cell. 1984;37:141–150. - PubMed
-
- Meeker TC, Nagarajan L, ar-Rushdi A, Rovera G, Huebner K, Croce CM. Characterization of the human PIM-1 gene: a putative proto-oncogene coding for a tissue specific member of the protein kinase family. Oncogene Res. 1987;1:87–101. - PubMed
-
- Ragoussis J, Senger G, Mockridge I, Sanseau P, Ruddy S, Dudley K, Sheer D, Trowsdale J. A testis-expressed Zn finger gene (ZNF76) in human 6p21.3 centromeric to the MHC is closely linked to the human homolog of the t-complex gene tcp-11. Genomics. 1992;14:673–679. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

