Tumor antigen ROR1 targeted drug delivery mediated selective leukemic but not normal B-cell cytotoxicity in chronic lymphocytic leukemia
- PMID: 24947019
- PMCID: PMC4272672
- DOI: 10.1038/leu.2014.199
Tumor antigen ROR1 targeted drug delivery mediated selective leukemic but not normal B-cell cytotoxicity in chronic lymphocytic leukemia
Abstract
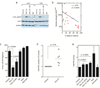
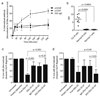
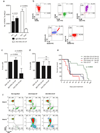
Selective cytotoxicity to cancer cells without compromising their normal counterparts pose a huge challenge for traditional drug design. Here we developed a tumor antigen-targeted delivery of immunonanoparticle carrying a novel non-immunosuppressive FTY720 derivative OSU-2S with potent cytotoxicity against leukemic B cells. OSU-2S induces activation of protein phosphatase 2A (PP2A), phosphorylation and nuclear translocation of SHP1(S591) and deregulation of multiple cellular processes in chronic lymphocytic leukemia (CLL) resulting in potent cytotoxicity. To preclude OSU-2S-mediated effects on these ubiquitous phosphatases in unintended cells and avoid potential adverse effects, we developed an OSU-2S-targeted delivery of immunonanoparticles (2A2-OSU-2S-ILP), that mediated selective cytotoxicity of CLL but not normal B cells through targeting receptor tyrosine kinase ROR1 expressed in leukemic but not normal B cells. Developing a novel spontaneous CLL mouse model expressing human ROR1 (hROR1) in all leukemic B cells, we demonstrate the therapeutic benefit of enhanced survival with 2A2-OSU-2S-ILP in vivo. The newly developed non-immunosuppressive OSU-2S, its delivery using human CLL directed immunonanoparticles and the novel transgenic (Tg) mouse model of CLL that expresses hROR1 exclusively in leukemic B cell surface are highly innovative and can be applied to CLL and other ROR1+ malignancies including mantle cell lymphoma and acute lymphoblastic leukemia.
Conflict of interest statement
Figures



Similar articles
-
ROR1-targeted delivery of OSU-2S, a nonimmunosuppressive FTY720 derivative, exerts potent cytotoxicity in mantle-cell lymphoma in vitro and in vivo.Exp Hematol. 2015 Sep;43(9):770-4.e2. doi: 10.1016/j.exphem.2015.04.008. Epub 2015 Apr 29. Exp Hematol. 2015. PMID: 25937048 Free PMC article.
-
FTY720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma.Blood. 2008 Jan 1;111(1):275-84. doi: 10.1182/blood-2006-10-053884. Epub 2007 Aug 29. Blood. 2008. PMID: 17761520 Free PMC article.
-
Targeting malignant B cells with an immunotoxin against ROR1.MAbs. 2012 May-Jun;4(3):349-61. doi: 10.4161/mabs.19870. Epub 2012 Apr 26. MAbs. 2012. PMID: 22531447 Free PMC article.
-
How receptor tyrosine kinase-like orphan receptor 1 meets its partners in chronic lymphocytic leukemia.Hematol Oncol. 2024 Mar;42(2):e3250. doi: 10.1002/hon.3250. Hematol Oncol. 2024. PMID: 38949887 Review.
-
ROR1: an orphan becomes apparent.Blood. 2022 Oct 6;140(14):1583-1591. doi: 10.1182/blood.2021014760. Blood. 2022. PMID: 35580162 Free PMC article. Review.
Cited by
-
Developing ROR1 Targeting CAR-T Cells against Solid Tumors in Preclinical Studies.Cancers (Basel). 2022 Jul 25;14(15):3618. doi: 10.3390/cancers14153618. Cancers (Basel). 2022. PMID: 35892876 Free PMC article.
-
Maintenance and pharmacologic targeting of ROR1 protein levels via UHRF1 in t(1;19) pre-B-ALL.Oncogene. 2018 Sep;37(38):5221-5232. doi: 10.1038/s41388-018-0299-8. Epub 2018 May 30. Oncogene. 2018. PMID: 29849118 Free PMC article.
-
Tyrosine Kinase ROR1 as a Target for Anti-Cancer Therapies.Front Oncol. 2021 May 28;11:680834. doi: 10.3389/fonc.2021.680834. eCollection 2021. Front Oncol. 2021. PMID: 34123850 Free PMC article. Review.
-
Pharmacokinetics and Tolerability of the Novel Non-immunosuppressive Fingolimod Derivative, OSU-2S, in Dogs and Comparisons with Data in Mice and Rats.AAPS J. 2020 Jul 16;22(4):92. doi: 10.1208/s12248-020-00474-9. AAPS J. 2020. PMID: 32676788 Free PMC article.
-
Human umbilical cord-derived mesenchymal stem cells protect against experimental colitis via CD5(+) B regulatory cells.Stem Cell Res Ther. 2016 Aug 11;7(1):109. doi: 10.1186/s13287-016-0376-2. Stem Cell Res Ther. 2016. PMID: 27515534 Free PMC article.
References
-
- Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. - PubMed
-
- Binet JL, Lepoprier M, Dighiero G, Charron D, D’Athis P, Vaugier G, et al. A clinical staging system for chronic lymphocytic leukemia: prognostic significance. Cancer. 1977;40:855–864. - PubMed
-
- Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S, et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell. 2005;8:355–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

