Regulation of HIV-Gag expression and targeting to the endolysosomal/secretory pathway by the luminal domain of lysosomal-associated membrane protein (LAMP-1) enhance Gag-specific immune response
- PMID: 24932692
- PMCID: PMC4059647
- DOI: 10.1371/journal.pone.0099887
Regulation of HIV-Gag expression and targeting to the endolysosomal/secretory pathway by the luminal domain of lysosomal-associated membrane protein (LAMP-1) enhance Gag-specific immune response
Abstract
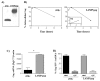
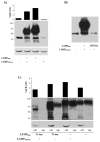
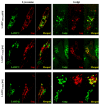
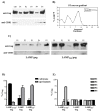
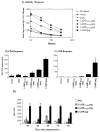
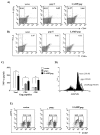
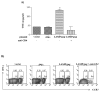
We have previously demonstrated that a DNA vaccine encoding HIV-p55gag in association with the lysosomal associated membrane protein-1 (LAMP-1) elicited a greater Gag-specific immune response, in comparison to a DNA encoding the native gag. In vitro studies have also demonstrated that LAMP/Gag was highly expressed and was present in MHCII containing compartments in transfected cells. In this study, the mechanisms involved in these processes and the relative contributions of the increased expression and altered traffic for the enhanced immune response were addressed. Cells transfected with plasmid DNA constructs containing p55gag attached to truncated sequences of LAMP-1 showed that the increased expression of gag mRNA required p55gag in frame with at least 741 bp of the LAMP-1 luminal domain. LAMP luminal domain also showed to be essential for Gag traffic through lysosomes and, in this case, the whole sequence was required. Further analysis of the trafficking pathway of the intact LAMP/Gag chimera demonstrated that it was secreted, at least in part, associated with exosome-like vesicles. Immunization of mice with LAMP/gag chimeric plasmids demonstrated that high expression level alone can induce a substantial transient antibody response, but targeting of the antigen to the endolysosomal/secretory pathways was required for establishment of cellular and memory response. The intact LAMP/gag construct induced polyfunctional CD4+ T cell response, which presence at the time of immunization was required for CD8+ T cell priming. LAMP-mediated targeting to endolysosomal/secretory pathway is an important new mechanistic element in LAMP-mediated enhanced immunity with applications to the development of novel anti-HIV vaccines and to general vaccinology field.
Conflict of interest statement
Figures

Similar articles
-
HIV-1 p55Gag encoded in the lysosome-associated membrane protein-1 as a DNA plasmid vaccine chimera is highly expressed, traffics to the major histocompatibility class II compartment, and elicits enhanced immune responses.J Biol Chem. 2003 Sep 26;278(39):37926-36. doi: 10.1074/jbc.M303336200. Epub 2003 Jun 24. J Biol Chem. 2003. PMID: 12824194
-
Inverted terminal repeat sequences of adeno-associated virus enhance the antibody and CD8(+) responses to a HIV-1 p55Gag/LAMP DNA vaccine chimera.Virology. 2004 Jun 1;323(2):220-32. doi: 10.1016/j.virol.2004.02.025. Virology. 2004. PMID: 15193918
-
Dendritic cells primed with a chimeric plasmid containing HIV-1-gag associated with lysosomal-associated protein-1 (LAMP/gag) is a potential therapeutic vaccine against HIV.FASEB J. 2016 Aug;30(8):2970-84. doi: 10.1096/fj.201500059. Epub 2016 May 19. FASEB J. 2016. PMID: 27199296
-
Dendritic cell-lysosomal-associated membrane protein (LAMP) and LAMP-1-HIV-1 gag chimeras have distinct cellular trafficking pathways and prime T and B cell responses to a diverse repertoire of epitopes.J Immunol. 2006 Aug 15;177(4):2265-75. doi: 10.4049/jimmunol.177.4.2265. J Immunol. 2006. PMID: 16887987
-
Endolysosomal vesicles at the center of B cell activation.J Cell Biol. 2024 Mar 4;223(3):e202307047. doi: 10.1083/jcb.202307047. Epub 2024 Feb 2. J Cell Biol. 2024. PMID: 38305771 Free PMC article. Review.
Cited by
-
Creation of an engineered APC system to explore and optimize the presentation of immunodominant peptides of major allergens.Sci Rep. 2016 Aug 19;6:31580. doi: 10.1038/srep31580. Sci Rep. 2016. PMID: 27539532 Free PMC article.
-
Molecular mechanisms for enhanced DNA vaccine immunogenicity.Expert Rev Vaccines. 2016;15(3):313-29. doi: 10.1586/14760584.2016.1124762. Epub 2015 Dec 28. Expert Rev Vaccines. 2016. PMID: 26707950 Free PMC article. Review.
-
Disparate Contributions of Human Retrovirus Capsid Subdomains to Gag-Gag Oligomerization, Virus Morphology, and Particle Biogenesis.J Virol. 2017 Jun 26;91(14):e00298-17. doi: 10.1128/JVI.00298-17. Print 2017 Jul 15. J Virol. 2017. PMID: 28446667 Free PMC article.
-
Critical Role of the Human T-Cell Leukemia Virus Type 1 Capsid N-Terminal Domain for Gag-Gag Interactions and Virus Particle Assembly.J Virol. 2018 Jun 29;92(14):e00333-18. doi: 10.1128/JVI.00333-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29695435 Free PMC article.
References
-
- Pontesilli O, Klein MR, Kerkhof-Garde SR, Pakker NG, de Wolf F, et al.. (1998) Longitudinal analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte responses: a predominant gag-specific response is associated with nonprogressive infection. J. Infect. Dis. 178(4): , 1008–1018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

