In vivo enrichment of genetically manipulated platelets corrects the murine hemophilic phenotype and induces immune tolerance even using a low multiplicity of infection
- PMID: 24931217
- PMCID: PMC4127102
- DOI: 10.1111/jth.12633
In vivo enrichment of genetically manipulated platelets corrects the murine hemophilic phenotype and induces immune tolerance even using a low multiplicity of infection
Abstract
Background: Our previous studies have demonstrated that platelet-specific gene delivery to hematopoietic stem cells can induce sustained therapeutic levels of platelet factor VIII (FVIII) expression in mice with hemophilia A.
Objective: In this study, we aimed to enhance platelet FVIII expression while minimizing potential toxicities.
Methods: A novel lentiviral vector (LV), which harbors dual genes, the FVIII gene driven by the αIIb promoter (2bF8) and a drug-resistance gene, the MGMT(P140K) cassette, was constructed. Platelet FVIII expression in mice with hemophilia A was introduced by transduction of hematopoietic stem cells and transplantation. The recipients were treated with O(6)-benzylguanine followed by 1,3-bis-2 chloroethyl-1-nitrosourea monthly three or four times. Animals were analyzed by using polymerase chain reaction (PCR), quantitative PCR, FVIII:C assays, and inhibitor assays. Phenotypic correction was assessed by tail clipping tests and rotational thromboelastometry analysis.
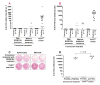
Results: Even using a low multiplicity of infection of 1 and a non-myeloablative conditioning regimen, after in vivo selection, the levels of platelet FVIII expression in recipients increased to 4.33 ± 5.48 mU per 10(8) platelets (n = 16), which were 19.7-fold higher than the levels obtained from the recipients before treatment. Quantitative PCR results confirmed that 2bF8/MGMT-LV-transduced cells were effectively enriched after drug-selective treatment. Fifteen of 16 treated animals survived tail clipping. Blood loss and whole blood clotting time were normalized in the treated recipients. Notably, no anti-FVIII antibodies were detected in the treated animals even after recombinant human B-domain deleted FVIII challenge.
Conclusion: we have established an effective in vivo selective system that allows us to enrich 2bF8LV-transduced cells, enhancing platelet FVIII expression while reducing the potential toxicities associated with platelet gene therapy.
Keywords: blood platelet; factor VIII; genetic therapy; hemophilia A; immune tolerance.
© 2014 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
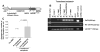
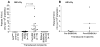
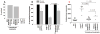
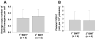
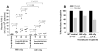

Similar articles
-
Immune tolerance induced by platelet-targeted factor VIII gene therapy in hemophilia A mice is CD4 T cell mediated.J Thromb Haemost. 2017 Oct;15(10):1994-2004. doi: 10.1111/jth.13800. Epub 2017 Sep 11. J Thromb Haemost. 2017. PMID: 28799202 Free PMC article.
-
In vivo enrichment of genetically manipulated platelets for murine hemophilia B gene therapy.J Cell Physiol. 2021 Jan;236(1):354-365. doi: 10.1002/jcp.29861. Epub 2020 Jun 8. J Cell Physiol. 2021. PMID: 32510630 Free PMC article.
-
The impact of GPIbα on platelet-targeted FVIII gene therapy in hemophilia A mice with pre-existing anti-FVIII immunity.J Thromb Haemost. 2019 Mar;17(3):449-459. doi: 10.1111/jth.14379. Epub 2019 Feb 3. J Thromb Haemost. 2019. PMID: 30609275 Free PMC article.
-
Hemophilia A gene therapy via intraosseous delivery of factor VIII-lentiviral vectors.Thromb J. 2016 Oct 4;14(Suppl 1):41. doi: 10.1186/s12959-016-0105-1. eCollection 2016. Thromb J. 2016. PMID: 27766066 Free PMC article. Review.
-
Platelet-Targeted FVIII Gene Therapy Restores Hemostasis and Induces Immune Tolerance for Hemophilia A.Front Immunol. 2020 Jun 12;11:964. doi: 10.3389/fimmu.2020.00964. eCollection 2020. Front Immunol. 2020. PMID: 32595633 Free PMC article. Review.
Cited by
-
Evaluating clinically translatable conditioning for platelet gene therapy in murine hemophilia A with inhibitors.J Thromb Haemost. 2024 Nov;22(11):3035-3047. doi: 10.1016/j.jtha.2024.07.023. Epub 2024 Aug 8. J Thromb Haemost. 2024. PMID: 39127324
-
Pre-existing anti-factor VIII immunity alters therapeutic platelet-targeted factor VIII engraftment following busulfan conditioning through cytotoxic CD8 T cells.J Thromb Haemost. 2023 Mar;21(3):488-498. doi: 10.1016/j.jtha.2022.10.006. Epub 2022 Dec 22. J Thromb Haemost. 2023. PMID: 36696197 Free PMC article.
-
Escape or Fight: Inhibitors in Hemophilia A.Front Immunol. 2020 Mar 24;11:476. doi: 10.3389/fimmu.2020.00476. eCollection 2020. Front Immunol. 2020. PMID: 32265927 Free PMC article. Review.
-
The impact of von Willebrand factor on factor VIII memory immune responses.Blood Adv. 2017 Aug 22;1(19):1565-1574. doi: 10.1182/bloodadvances.2017009209. Epub 2017 Aug 18. Blood Adv. 2017. PMID: 28920105 Free PMC article.
-
Alloantibodies to therapeutic factor VIII in hemophilia A: the role of von Willebrand factor in regulating factor VIII immunogenicity.Haematologica. 2015 Feb;100(2):149-56. doi: 10.3324/haematol.2014.112821. Haematologica. 2015. PMID: 25638804 Free PMC article. Review.
References
-
- High KA. Gene transfer as an approach to treating hemophilia. Semin Thromb Hemost. 2003;29:107–20. - PubMed
-
- High KA. Gene therapy for haemophilia: a long and winding road. J Thromb Haemost. 2011;9 (Suppl 1):2–11. - PubMed
-
- Saenko EL, Ananyeva NM, Moayeri M, Ramezani A, Hawley RG. Development of improved factor VIII molecules and new gene transfer approaches for hemophilia A. Curr Gene Ther. 2003;3:27–41. - PubMed
-
- Balague C, Zhou J, Dai Y, Alemany R, Josephs SF, Andreason G, Hariharan M, Sethi E, Prokopenko E, Jan HY, Lou YC, Hubert-Leslie D, Ruiz L, Zhang WW. Sustained high-level expression of full-length human factor VIII and restoration of clotting activity in hemophilic mice using a minimal adenovirus vector. Blood. 2000;95:820–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

