Hydrolyzed infant formula and early β-cell autoimmunity: a randomized clinical trial
- PMID: 24915259
- PMCID: PMC4225544
- DOI: 10.1001/jama.2014.5610
Hydrolyzed infant formula and early β-cell autoimmunity: a randomized clinical trial
Abstract
Importance: The disease process leading to clinical type 1 diabetes often starts during the first years of life. Early exposure to complex dietary proteins may increase the risk of β-cell autoimmunity in children at genetic risk for type 1 diabetes. Extensively hydrolyzed formulas do not contain intact proteins.
Objective: To test the hypothesis that weaning to an extensively hydrolyzed formula decreases the cumulative incidence of diabetes-associated autoantibodies in young children.
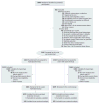
Design, setting, and participants: A double-blind randomized clinical trial of 2159 infants with HLA-conferred disease susceptibility and a first-degree relative with type 1 diabetes recruited from May 2002 to January 2007 in 78 study centers in 15 countries; 1078 were randomized to be weaned to the extensively hydrolyzed casein formula and 1081 were randomized to be weaned to a conventional cows' milk-based formula. The participants were observed to April 16, 2013.
Interventions: The participants received either a casein hydrolysate or a conventional cows' milk formula supplemented with 20% of the casein hydrolysate.
Main outcomes: AND MEASURES: Primary outcome was positivity for at least 2 diabetes-associated autoantibodies out of 4 analyzed. Autoantibodies to insulin, glutamic acid decarboxylase, and the insulinoma-associated-2 (IA-2) molecule were analyzed using radiobinding assays and islet cell antibodies with immunofluorescence during a median observation period of 7.0 years (mean, 6.3 years).
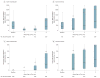
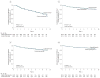
Results: The absolute risk of positivity for 2 or more islet autoantibodies was 13.4% among those randomized to the casein hydrolysate formula (n = 139) vs 11.4% among those randomized to the conventional formula (n = 117). The unadjusted hazard ratio for positivity for 2 or more autoantibodies among those randomized to be weaned to the casein hydrolysate was 1.21 (95% CI, 0.94-1.54), compared with those randomized to the conventional formula, while the hazard ratio adjusted for HLA risk, duration of breastfeeding, vitamin D use, study formula duration and consumption, and region was 1.23 (95% CI, 0.96-1.58). There were no clinically significant differences in the rate of reported adverse events between the 2 groups.
Conclusions and relevance: Among infants at risk for type 1 diabetes, the use of a hydrolyzed formula, when compared with a conventional formula, did not reduce the incidence of diabetes-associated autoantibodies after 7 years. These findings do not support a benefit from hydrolyzed formula. TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT00179777.
Conflict of interest statement
Figures
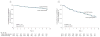
Similar articles
-
Effect of Hydrolyzed Infant Formula vs Conventional Formula on Risk of Type 1 Diabetes: The TRIGR Randomized Clinical Trial.JAMA. 2018 Jan 2;319(1):38-48. doi: 10.1001/jama.2017.19826. JAMA. 2018. PMID: 29297078 Free PMC article. Clinical Trial.
-
Dietary intervention in infancy and later signs of beta-cell autoimmunity.N Engl J Med. 2010 Nov 11;363(20):1900-8. doi: 10.1056/NEJMoa1004809. N Engl J Med. 2010. PMID: 21067382 Free PMC article. Clinical Trial.
-
Avoidance of Cow's Milk-Based Formula for At-Risk Infants Does Not Reduce Development of Celiac Disease: A Randomized Controlled Trial.Gastroenterology. 2017 Oct;153(4):961-970.e3. doi: 10.1053/j.gastro.2017.06.049. Epub 2017 Jul 5. Gastroenterology. 2017. PMID: 28687275 Clinical Trial.
-
Early feeding and risk of type 1 diabetes: experiences from the Trial to Reduce Insulin-dependent diabetes mellitus in the Genetically at Risk (TRIGR).Am J Clin Nutr. 2011 Dec;94(6 Suppl):1814S-1820S. doi: 10.3945/ajcn.110.000711. Epub 2011 Jun 8. Am J Clin Nutr. 2011. PMID: 21653795 Free PMC article. Review.
-
Formulas containing hydrolysed protein for prevention of allergy and food intolerance in infants.Cochrane Database Syst Rev. 2006 Oct 18;(4):CD003664. doi: 10.1002/14651858.CD003664.pub3. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2017 Mar 15;3:CD003664. doi: 10.1002/14651858.CD003664.pub4. PMID: 17054180 Updated. Review.
Cited by
-
Association of environmental markers with childhood type 1 diabetes mellitus revealed by a long questionnaire on early life exposures and lifestyle in a case-control study.BMC Public Health. 2016 Sep 29;16(1):1021. doi: 10.1186/s12889-016-3690-9. BMC Public Health. 2016. PMID: 27682602 Free PMC article.
-
Islet Autoantibodies.Curr Diab Rep. 2016 Jun;16(6):53. doi: 10.1007/s11892-016-0738-2. Curr Diab Rep. 2016. PMID: 27112957 Review.
-
Infant formulas containing hydrolysed protein for prevention of allergic disease.Cochrane Database Syst Rev. 2018 Oct 19;10(10):CD003664. doi: 10.1002/14651858.CD003664.pub6. Cochrane Database Syst Rev. 2018. PMID: 30338526 Free PMC article.
-
Type 1 Diabetes at-risk children highly recognize Mycobacterium avium subspecies paratuberculosis epitopes homologous to human Znt8 and Proinsulin.Sci Rep. 2016 Feb 29;6:22266. doi: 10.1038/srep22266. Sci Rep. 2016. PMID: 26923214 Free PMC article.
-
Early life origin of type 1 diabetes.Semin Immunopathol. 2017 Nov;39(6):653-667. doi: 10.1007/s00281-017-0665-6. Epub 2017 Nov 23. Semin Immunopathol. 2017. PMID: 29170800 Review.
References
-
- Knip M. Natural course of preclinical type 1 diabetes. Horm Res. 2002;57(suppl 1):6–11. - PubMed
-
- Knip M. Can we predict type 1 diabetes in the general population? Diabetes Care. 2002;25(3):623–625. - PubMed
-
- Ziegler A-G, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes. 1999;48 (3):460–468. - PubMed
-
- Kimpimäki T, Kupila A, Hämäläinen A-M, et al. The first signs of β-cell autoimmunity appear in infancy in genetically susceptible children from the general population: the Finnish Type 1 Diabetes Prediction and Prevention Study. J Clin Endocrinol Metab. 2001;86(10):4782–4788. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

