Population pharmacokinetic modeling of LY2189102 after multiple intravenous and subcutaneous administrations
- PMID: 24912797
- PMCID: PMC4147062
- DOI: 10.1208/s12248-014-9623-6
Population pharmacokinetic modeling of LY2189102 after multiple intravenous and subcutaneous administrations
Abstract
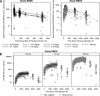
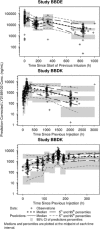
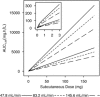
Interleukin-1 beta (IL-1β) is an inflammatory mediator which may contribute to the pathophysiology of rheumatoid arthritis (RA) and type 2 diabetes mellitus (T2DM). Population pharmacokinetics (PK) of LY2189102, a high affinity anti-IL-1β humanized monoclonal immunoglobulin G4 evaluated for efficacy in RA and T2DM, were characterized using data from 79 T2DM subjects (Study H9C-MC-BBDK) who received 13 weekly subcutaneous (SC) doses of LY2189102 (0.6, 18, and 180 mg) and 96 RA subjects (Study H9C-MC-BBDE) who received five weekly intravenous (IV) doses (0.02-2.5 mg/kg). Frequency of anti-drug antibody (ADA) development appears dose-dependent and is different between studies (36.7% in Study H9C-MC-BBDK vs. 2.1% in Study H9C-MC-BBDE), likely due to several factors, including differences in patient population and background medications, administration routes, and assays. A two-compartment model with dose-dependent bioavailability best characterizes LY2189102 PK following IV and SC administration. Typical elimination and distribution clearances, central and peripheral volumes of distribution are 0.222 L/day, 0.518 L/day, 3.08 L, and 1.94 L, resulting in a terminal half-life of 16.8 days. Elimination clearance increased linearly, yet modestly, with baseline creatinine clearance and appears 37.6% higher in subjects who developed ADA. Bioavailability (0.432-0.721) and absorption half-life (94.3-157 h) after SC administration are smaller with larger doses. Overall, LY2189102 PK is consistent with other therapeutic humanized monoclonal antibodies and is likely to support convenient SC dosing.
Trial registration: ClinicalTrials.gov NCT00380744 NCT00942188.
Figures

Similar articles
-
Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1β antibody, in patients with type 2 diabetes.Diabetes Care. 2013 Aug;36(8):2239-46. doi: 10.2337/dc12-1835. Epub 2013 Mar 20. Diabetes Care. 2013. PMID: 23514733 Free PMC article. Clinical Trial.
-
Safety and pharmacokinetics of olokizumab, an anti-IL-6 monoclonal antibody, administered to healthy male volunteers: A randomized phase I study.Clin Pharmacol Drug Dev. 2014 Sep;3(5):388-95. doi: 10.1002/cpdd.121. Epub 2014 May 26. Clin Pharmacol Drug Dev. 2014. PMID: 27129012 Clinical Trial.
-
Comparison of intravenous and subcutaneous exposure supporting dose selection of subcutaneous belimumab systemic lupus erythematosus Phase 3 program.Lupus. 2016 Nov;25(13):1448-1455. doi: 10.1177/0961203316642309. Epub 2016 Jul 11. Lupus. 2016. PMID: 27072354 Free PMC article. Clinical Trial.
-
Drug delivery options to increase patient adherence and satisfaction in the management of rheumatoid arthritis -- focus on subcutaneous tocilizumab.Drug Des Devel Ther. 2014 Jul 4;8:913-9. doi: 10.2147/DDDT.S52099. eCollection 2014. Drug Des Devel Ther. 2014. PMID: 25045250 Free PMC article. Review.
-
Safety of subcutaneous versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with rheumatoid arthritis.Expert Opin Drug Saf. 2015 Mar;14(3):429-37. doi: 10.1517/14740338.2015.998198. Epub 2015 Jan 2. Expert Opin Drug Saf. 2015. PMID: 25553607 Review.
Cited by
-
The Role of NLRP3 Inflammasome in Pneumococcal Infections.Front Immunol. 2020 Dec 14;11:614801. doi: 10.3389/fimmu.2020.614801. eCollection 2020. Front Immunol. 2020. PMID: 33424869 Free PMC article. Review.
-
The role of interleukin-1 in general pathology.Inflamm Regen. 2019 Jun 6;39:12. doi: 10.1186/s41232-019-0101-5. eCollection 2019. Inflamm Regen. 2019. PMID: 31182982 Free PMC article. Review.
-
Potential of IL-1, IL-18 and Inflammasome Inhibition for the Treatment of Inflammatory Skin Diseases.Front Pharmacol. 2017 May 22;8:278. doi: 10.3389/fphar.2017.00278. eCollection 2017. Front Pharmacol. 2017. PMID: 28588486 Free PMC article. Review.
-
A decade of innovation in pharmaceutical R&D: the Chorus model.Nat Rev Drug Discov. 2015 Jan;14(1):17-28. doi: 10.1038/nrd4497. Epub 2014 Dec 15. Nat Rev Drug Discov. 2015. PMID: 25503514 Review.
-
Necroinflammation in Kidney Disease.J Am Soc Nephrol. 2016 Jan;27(1):27-39. doi: 10.1681/ASN.2015040405. Epub 2015 Sep 2. J Am Soc Nephrol. 2016. PMID: 26334031 Free PMC article. Review.
References
-
- Kineret (anakinra) [package insert]. Stockholm, Sweden: Swedish Orphan Biovitrum AB; 2012. http://www.kineretrx.com/fileadmin/user_upload/kineretus/documents/Kiner.... Published Dec 2012. Accessed 20 Feb 2014.
-
- Kineret (anakinra). Summary of product characteristics. 2002. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info.... Accessed 20 Feb 2014.
-
- Donath MY, Weder C, Whitmore J, Bauer RJ, Der K, Scannon PJ, et al. XOMA 052, an anti–IL–1β antibody, in a double-blind, placebo-controlled, dose escalation study of the safety and pharmacokinetics in patients with type 2 diabetes mellitus—a new approach to therapy. Diabetologia. 2008;51(Suppl 1):S7.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

