Brachypodium distachyon as a model system for studies of copper transport in cereal crops
- PMID: 24910638
- PMCID: PMC4039008
- DOI: 10.3389/fpls.2014.00236
Brachypodium distachyon as a model system for studies of copper transport in cereal crops
Abstract
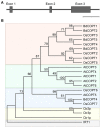
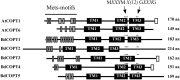
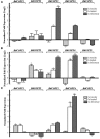
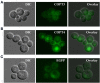
Copper (Cu) is an essential micronutrient that performs a remarkable array of functions in plants including photosynthesis, cell wall remodeling, flowering, and seed set. Of the world's major cereal crops, wheat, barley, and oat are the most sensitive to Cu deficiency. Cu deficient soils include alkaline soils, which occupy approximately 30% of the world's arable lands, and organic soils that occupy an estimated 19% of arable land in Europe. We used Brachypodium distachyon (brachypodium) as a proxy for wheat and other grain cereals to initiate analyses of the molecular mechanisms underlying their increased susceptibility to Cu deficiency. In this report, we focus on members of the CTR/COPT family of Cu transporters because their homologs in A. thaliana are transcriptionally upregulated in Cu-limited conditions and are involved either in Cu uptake from soils into epidermal cells in the root, or long-distance transport and distribution of Cu in photosynthetic tissues. We found that of five COPT proteins in brachypodium, BdCOPT3, and BdCOPT4 localize to the plasma membrane and are transcriptionally upregulated in roots and leaves by Cu deficiency. We also found that BdCOPT3, BdCOPT4, and BdCOPT5 confer low affinity Cu transport, in contrast to their counterparts in A. thaliana that confer high affinity Cu transport. These data suggest that increased sensitivity to Cu deficiency in some grass species may arise from lower efficiency and, possibly, other properties of components of Cu uptake and tissue partitioning systems and reinforce the importance of using brachypodium as a model for the comprehensive analyses of Cu homeostasis in cereal crops.
Keywords: Brachypodium; CTR/COPT transporters; copper homeostasis; copper transport; wheat.
Figures



Similar articles
-
The CTR/COPT-dependent copper uptake and SPL7-dependent copper deficiency responses are required for basal cadmium tolerance in A. thaliana.Metallomics. 2013 Sep;5(9):1262-75. doi: 10.1039/c3mt00111c. Metallomics. 2013. PMID: 23835944
-
The shoot and root growth of Brachypodium and its potential as a model for wheat and other cereal crops.Funct Plant Biol. 2009 Nov;36(11):960-969. doi: 10.1071/FP09214. Funct Plant Biol. 2009. PMID: 32688707
-
The Arabidopsis COPT6 transport protein functions in copper distribution under copper-deficient conditions.Plant Cell Physiol. 2013 Aug;54(8):1378-90. doi: 10.1093/pcp/pct088. Epub 2013 Jun 12. Plant Cell Physiol. 2013. PMID: 23766354
-
Brachypodium: a promising hub between model species and cereals.J Exp Bot. 2014 Oct;65(19):5683-96. doi: 10.1093/jxb/eru376. J Exp Bot. 2014. PMID: 25262566 Review.
-
Molecular Mechanisms of Plant Responses to Copper: From Deficiency to Excess.Int J Mol Sci. 2024 Jun 26;25(13):6993. doi: 10.3390/ijms25136993. Int J Mol Sci. 2024. PMID: 39000099 Free PMC article. Review.
Cited by
-
Cupid, a cell permeable peptide derived from amoeba, capable of delivering GFP into a diverse range of species.Sci Rep. 2020 Aug 13;10(1):13725. doi: 10.1038/s41598-020-70532-x. Sci Rep. 2020. PMID: 32792509 Free PMC article.
-
In Vitro Tissue Culture in Brachypodium: Applications and Challenges.Int J Mol Sci. 2020 Feb 4;21(3):1037. doi: 10.3390/ijms21031037. Int J Mol Sci. 2020. PMID: 32033195 Free PMC article. Review.
-
YSL3-mediated copper distribution is required for fertility, seed size and protein accumulation in Brachypodium.Plant Physiol. 2021 May 27;186(1):655-676. doi: 10.1093/plphys/kiab054. Plant Physiol. 2021. PMID: 33576792 Free PMC article.
-
Are Grasses Really Useful for the Phytoremediation of Potentially Toxic Trace Elements? A Review.Front Plant Sci. 2021 Nov 24;12:778275. doi: 10.3389/fpls.2021.778275. eCollection 2021. Front Plant Sci. 2021. PMID: 34917111 Free PMC article. Review.
-
From soil to seed: micronutrient movement into and within the plant.Front Plant Sci. 2014 Sep 5;5:438. doi: 10.3389/fpls.2014.00438. eCollection 2014. Front Plant Sci. 2014. PMID: 25250035 Free PMC article. No abstract available.
References
-
- Arteca R. N., Arteca J. M. (2000). A novel method for growing Arabidopsis thaliana plants hydroponically. Physiol. Plantarum 108, 188–193 10.1034/j.1399-3054.2000.108002188.x - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

