How epigallocatechin gallate can inhibit α-synuclein oligomer toxicity in vitro
- PMID: 24907278
- PMCID: PMC4118093
- DOI: 10.1074/jbc.M114.554667
How epigallocatechin gallate can inhibit α-synuclein oligomer toxicity in vitro
Abstract
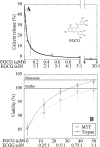
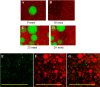
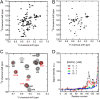
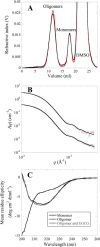
Oligomeric species of various proteins are linked to the pathogenesis of different neurodegenerative disorders. Consequently, there is intense focus on the discovery of novel inhibitors, e.g. small molecules and antibodies, to inhibit the formation and block the toxicity of oligomers. In Parkinson disease, the protein α-synuclein (αSN) forms cytotoxic oligomers. The flavonoid epigallocatechin gallate (EGCG) has previously been shown to redirect the aggregation of αSN monomers and remodel αSN amyloid fibrils into disordered oligomers. Here, we dissect EGCG's mechanism of action. EGCG inhibits the ability of preformed oligomers to permeabilize vesicles and induce cytotoxicity in a rat brain cell line. However, EGCG does not affect oligomer size distribution or secondary structure. Rather, EGCG immobilizes the C-terminal region and moderately reduces the degree of binding of oligomers to membranes. We interpret our data to mean that the oligomer acts by destabilizing the membrane rather than by direct pore formation. This suggests that reduction (but not complete abolition) of the membrane affinity of the oligomer is sufficient to prevent cytotoxicity.
Keywords: Dynamic Light Scattering; EGCG; Isothermal Titration Calorimetry (ITC); Membrane; Nuclear Magnetic Resonance (NMR); Oligomer; Toxicity; X-ray Scattering; α-Synuclein.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
How epigallocatechin gallate binds and assembles oligomeric forms of human alpha-synuclein.J Biol Chem. 2021 Jan-Jun;296:100788. doi: 10.1016/j.jbc.2021.100788. Epub 2021 May 18. J Biol Chem. 2021. PMID: 34019875 Free PMC article.
-
EGCG-mediated Protection of the Membrane Disruption and Cytotoxicity Caused by the 'Active Oligomer' of α-Synuclein.Sci Rep. 2017 Dec 20;7(1):17945. doi: 10.1038/s41598-017-18349-z. Sci Rep. 2017. PMID: 29263416 Free PMC article.
-
The N-terminus of α-synuclein is essential for both monomeric and oligomeric interactions with membranes.FEBS Lett. 2014 Jan 31;588(3):497-502. doi: 10.1016/j.febslet.2013.12.015. Epub 2013 Dec 25. FEBS Lett. 2014. PMID: 24374342
-
In Vitro and In Silico Studies of the Molecular Interactions of Epigallocatechin-3-O-gallate (EGCG) with Proteins That Explain the Health Benefits of Green Tea.Molecules. 2018 May 28;23(6):1295. doi: 10.3390/molecules23061295. Molecules. 2018. PMID: 29843451 Free PMC article. Review.
-
Polymorphism in alpha-synuclein oligomers and its implications in toxicity under disease conditions.Front Mol Biosci. 2022 Aug 12;9:959425. doi: 10.3389/fmolb.2022.959425. eCollection 2022. Front Mol Biosci. 2022. PMID: 36032665 Free PMC article. Review.
Cited by
-
Polyphenol-solubility alters amyloid fibril formation of α-synuclein.Protein Sci. 2021 Aug;30(8):1701-1713. doi: 10.1002/pro.4130. Epub 2021 Jun 2. Protein Sci. 2021. PMID: 34046949 Free PMC article.
-
Toxic Oligomeric Alpha-Synuclein Variants Present in Human Parkinson's Disease Brains Are Differentially Generated in Mammalian Cell Models.Biomolecules. 2015 Jul 22;5(3):1634-51. doi: 10.3390/biom5031634. Biomolecules. 2015. PMID: 26287258 Free PMC article.
-
Resistance, Tolerance, Virulence and Bacterial Pathogen Fitness-Current State and Envisioned Solutions for the Near Future.Pathogens. 2023 May 22;12(5):746. doi: 10.3390/pathogens12050746. Pathogens. 2023. PMID: 37242416 Free PMC article. Review.
-
Epigallocatechin-3-gallate preferentially induces aggregation of amyloidogenic immunoglobulin light chains.Sci Rep. 2017 Jan 27;7:41515. doi: 10.1038/srep41515. Sci Rep. 2017. PMID: 28128355 Free PMC article.
-
Cellular Stress Response (Hormesis) in Response to Bioactive Nutraceuticals with Relevance to Alzheimer Disease.Antioxid Redox Signal. 2023 Mar;38(7-9):643-669. doi: 10.1089/ars.2022.0214. Antioxid Redox Signal. 2023. PMID: 36656673 Free PMC article. Review.
References
-
- Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. (1997) α-Synuclein in Lewy bodies. Nature 388, 839–840 - PubMed
-
- Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E. S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W. G., Lazzarini A. M., Duvoisin R. C., Di Iorio G., Golbe L. I., Nussbaum R. L. (1997) Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 - PubMed
-
- Conway K. A., Harper J. D., Lansbury P. T. (1998) Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat. Med. 4, 1318–1320 - PubMed
-
- Narhi L., Wood S. J., Steavenson S., Jiang Y., Wu G. M., Anafi D., Kaufman S. A., Martin F., Sitney K., Denis P., Louis J. C., Wypych J., Biere A. L., Citron M. (1999) Both familial Parkinson's disease mutations accelerate α-synuclein aggregation. J. Biol. Chem. 274, 9843–9846 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

