Viral phosphodiesterases that antagonize double-stranded RNA signaling to RNase L by degrading 2-5A
- PMID: 24905202
- PMCID: PMC4046343
- DOI: 10.1089/jir.2014.0007
Viral phosphodiesterases that antagonize double-stranded RNA signaling to RNase L by degrading 2-5A
Abstract
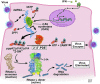
The host interferon (IFN) antiviral response involves a myriad of diverse biochemical pathways that disrupt virus replication cycles at many different levels. As a result, viruses have acquired and evolved genes that antagonize the host antiviral proteins. IFNs inhibit viral infections in part through the 2',5'-oligoadenylate (2-5A) synthetase (OAS)/RNase L pathway. OAS proteins are pathogen recognition receptors that exist at different basal levels in different cell types and that are IFN inducible. Upon activation by the pathogen-associated molecular pattern viral double-stranded RNA, certain OAS proteins synthesize 2-5A from ATP. 2-5A binds to the antiviral enzyme RNase L causing its dimerization and activation. Recently, disparate RNA viruses, group 2a betacoronaviruses, and group A rotaviruses, have been shown to produce proteins with 2',5'-phosphodiesterase (PDE) activities that eliminate 2-5A thereby evading the antiviral activity of the OAS/RNase L pathway. These viral proteins are members of the eukaryotic-viral LigT-like group of 2H phosphoesterases, so named for the presence of 2 conserved catalytic histidine residues. Here, we will review the biochemistry, biology, and implications of viral and cellular 2',5'-PDEs that degrade 2-5A. In addition, we discuss alternative viral and cellular strategies for limiting the activity of OAS/RNase L.
Figures

Similar articles
-
Homologous 2',5'-phosphodiesterases from disparate RNA viruses antagonize antiviral innate immunity.Proc Natl Acad Sci U S A. 2013 Aug 6;110(32):13114-9. doi: 10.1073/pnas.1306917110. Epub 2013 Jul 22. Proc Natl Acad Sci U S A. 2013. PMID: 23878220 Free PMC article.
-
Cell-type-specific effects of RNase L on viral induction of beta interferon.mBio. 2014 Feb 25;5(2):e00856-14. doi: 10.1128/mBio.00856-14. mBio. 2014. PMID: 24570368 Free PMC article.
-
Activation of RNase L by Murine Coronavirus in Myeloid Cells Is Dependent on Basal Oas Gene Expression and Independent of Virus-Induced Interferon.J Virol. 2016 Jan 6;90(6):3160-72. doi: 10.1128/JVI.03036-15. J Virol. 2016. PMID: 26739051 Free PMC article.
-
New insights into the role of RNase L in innate immunity.J Interferon Cytokine Res. 2011 Jan;31(1):49-57. doi: 10.1089/jir.2010.0120. Epub 2010 Dec 29. J Interferon Cytokine Res. 2011. PMID: 21190483 Free PMC article. Review.
-
The Many Faces of Oligoadenylate Synthetases.J Interferon Cytokine Res. 2023 Nov;43(11):487-494. doi: 10.1089/jir.2023.0098. Epub 2023 Sep 25. J Interferon Cytokine Res. 2023. PMID: 37751211 Free PMC article. Review.
Cited by
-
Enzyme assays for synthesis and degradation of 2-5As and other 2'-5' oligonucleotides.BMC Biochem. 2015 Jun 26;16:15. doi: 10.1186/s12858-015-0043-8. BMC Biochem. 2015. PMID: 26113370 Free PMC article.
-
Occludin Regulates HIV-1 Infection by Modulation of the Interferon Stimulated OAS Gene Family.Mol Neurobiol. 2023 Sep;60(9):4966-4982. doi: 10.1007/s12035-023-03381-0. Epub 2023 May 20. Mol Neurobiol. 2023. PMID: 37209263 Free PMC article.
-
Genetic mechanisms of critical illness in COVID-19.Nature. 2021 Mar;591(7848):92-98. doi: 10.1038/s41586-020-03065-y. Epub 2020 Dec 11. Nature. 2021. PMID: 33307546
-
In vitro and bioinformatics mechanistic-based approach for cadmium carcinogenicity understanding.Toxicol In Vitro. 2020 Jun;65:104757. doi: 10.1016/j.tiv.2020.104757. Epub 2020 Jan 3. Toxicol In Vitro. 2020. PMID: 31904401 Free PMC article.
-
Occludin regulates HIV-1 infection by modulation of the interferon stimulated OAS gene family.Res Sq [Preprint]. 2023 Jan 30:rs.3.rs-2501091. doi: 10.21203/rs.3.rs-2501091/v1. Res Sq. 2023. Update in: Mol Neurobiol. 2023 Sep;60(9):4966-4982. doi: 10.1007/s12035-023-03381-0 PMID: 36778388 Free PMC article. Updated. Preprint.
References
-
- Arn EA, Abelson JN. 1996. The 2′-5′ RNA ligase of Escherichia coli. Purification, cloning, and genomic disruption. J Biol Chem 271:31145–31153 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

