Heme on innate immunity and inflammation
- PMID: 24904418
- PMCID: PMC4035012
- DOI: 10.3389/fphar.2014.00115
Heme on innate immunity and inflammation
Abstract
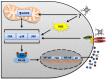
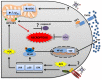
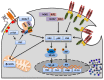
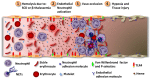
Heme is an essential molecule expressed ubiquitously all through our tissues. Heme plays major functions in cellular physiology and metabolism as the prosthetic group of diverse proteins. Once released from cells and from hemeproteins free heme causes oxidative damage and inflammation, thus acting as a prototypic damage-associated molecular pattern. In this context, free heme is a critical component of the pathological process of sterile and infectious hemolytic conditions including malaria, hemolytic anemias, ischemia-reperfusion, and hemorrhage. The plasma scavenger proteins hemopexin and albumin reduce heme toxicity and are responsible for transporting free heme to intracellular compartments where it is catabolized by heme-oxygenase enzymes. Upon hemolysis or severe cellular damage the serum capacity to scavenge heme may saturate and increase free heme to sufficient amounts to cause tissue damage in various organs. The mechanism by which heme causes reactive oxygen generation, activation of cells of the innate immune system and cell death are not fully understood. Although heme can directly promote lipid peroxidation by its iron atom, heme can also induce reactive oxygen species generation and production of inflammatory mediators through the activation of selective signaling pathways. Heme activates innate immune cells such as macrophages and neutrophils through activation of innate immune receptors. The importance of these events has been demonstrated in infectious and non-infectious diseases models. In this review, we will discuss the mechanisms behind heme-induced cytotoxicity and inflammation and the consequences of these events on different tissues and diseases.
Keywords: ROS; cytotoxicity; heme; hemolysis; inflammation; innate immunity; iron; programed cell death.
Figures
Similar articles
-
The different facets of heme-oxygenase 1 in innate and adaptive immunity.Cell Biochem Biophys. 2022 Dec;80(4):609-631. doi: 10.1007/s12013-022-01087-z. Epub 2022 Aug 26. Cell Biochem Biophys. 2022. PMID: 36018440 Review.
-
Protein aggregation as a cellular response to oxidative stress induced by heme and iron.Proc Natl Acad Sci U S A. 2016 Nov 22;113(47):E7474-E7482. doi: 10.1073/pnas.1608928113. Epub 2016 Nov 7. Proc Natl Acad Sci U S A. 2016. PMID: 27821769 Free PMC article.
-
Heme and hemolysis in innate immunity: adding insult to injury.Curr Opin Immunol. 2018 Feb;50:14-20. doi: 10.1016/j.coi.2017.10.005. Epub 2017 Nov 5. Curr Opin Immunol. 2018. PMID: 29107115 Review.
-
Heme as a danger molecule in pathogen recognition.Free Radic Biol Med. 2015 Dec;89:651-61. doi: 10.1016/j.freeradbiomed.2015.08.020. Epub 2015 Oct 9. Free Radic Biol Med. 2015. PMID: 26456060 Review.
-
Heme and iron induce protein aggregation.Autophagy. 2017 Mar 4;13(3):625-626. doi: 10.1080/15548627.2016.1271515. Epub 2017 Jan 5. Autophagy. 2017. PMID: 28055290 Free PMC article.
Cited by
-
Immunoregulation role of the erythroid cells.Front Immunol. 2024 Oct 15;15:1466669. doi: 10.3389/fimmu.2024.1466669. eCollection 2024. Front Immunol. 2024. PMID: 39474425 Free PMC article. Review.
-
Chemical Composition and Antioxidant Activity of Six Allium Extracts Using Protein-Based Biomimetic Methods.Antioxidants (Basel). 2024 Sep 29;13(10):1182. doi: 10.3390/antiox13101182. Antioxidants (Basel). 2024. PMID: 39456436 Free PMC article.
-
Comprehensive time-course gene expression evaluation of high-risk beef cattle to establish immunological characteristics associated with undifferentiated bovine respiratory disease.Front Immunol. 2024 Sep 13;15:1412766. doi: 10.3389/fimmu.2024.1412766. eCollection 2024. Front Immunol. 2024. PMID: 39346910 Free PMC article.
-
Therapeutics for sickle cell disease intravascular hemolysis.Front Physiol. 2024 Sep 13;15:1474569. doi: 10.3389/fphys.2024.1474569. eCollection 2024. Front Physiol. 2024. PMID: 39345787 Free PMC article. Review.
-
Circulating free heme induces cytokine storm and pulmonary hypertension through the MKK3/p38 axis.Am J Physiol Lung Cell Mol Physiol. 2024 Oct 1;327(4):L574-L586. doi: 10.1152/ajplung.00422.2022. Epub 2024 Aug 28. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 39197168 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

