Directed midbrain and spinal cord neurogenesis from pluripotent stem cells to model development and disease in a dish
- PMID: 24904255
- PMCID: PMC4033221
- DOI: 10.3389/fnins.2014.00109
Directed midbrain and spinal cord neurogenesis from pluripotent stem cells to model development and disease in a dish
Abstract
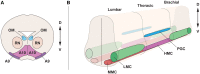
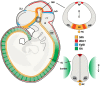
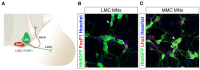
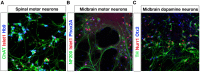
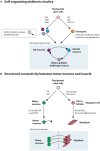
Induction of specific neuronal fates is restricted in time and space in the developing CNS through integration of extrinsic morphogen signals and intrinsic determinants. Morphogens impose regional characteristics on neural progenitors and establish distinct progenitor domains. Such domains are defined by unique expression patterns of fate determining transcription factors. These processes of neuronal fate specification can be recapitulated in vitro using pluripotent stem cells. In this review, we focus on the generation of dopamine neurons and motor neurons, which are induced at ventral positions of the neural tube through Sonic hedgehog (Shh) signaling, and defined at anteroposterior positions by fibroblast growth factor (Fgf) 8, Wnt1, and retinoic acid (RA). In vitro utilization of these morphogenic signals typically results in the generation of multiple neuronal cell types, which are defined at the intersection of these signals. If the purpose of in vitro neurogenesis is to generate one cell type only, further lineage restriction can be accomplished by forced expression of specific transcription factors in a permissive environment. Alternatively, cell-sorting strategies allow for selection of neuronal progenitors or mature neurons. However, modeling development, disease and prospective therapies in a dish could benefit from structured heterogeneity, where desired neurons are appropriately synaptically connected and thus better reflect the three-dimensional structure of that region. By modulating the extrinsic environment to direct sequential generation of neural progenitors within a domain, followed by self-organization and synaptic establishment, a reductionist model of that brain region could be created. Here we review recent advances in neuronal fate induction in vitro, with a focus on the interplay between cell intrinsic and extrinsic factors, and discuss the implications for studying development and disease in a dish.
Keywords: Parkinson disease; amyotrophic lateral sclerosis; dopamine neuron; in vitro neuronal networks; motor neuron; stem cells.
Figures

Similar articles
-
Heterogeneity in the developmental potential of motor neuron progenitors revealed by clonal analysis of single cells in vitro.Neural Dev. 2009 Jan 5;4:2. doi: 10.1186/1749-8104-4-2. Neural Dev. 2009. PMID: 19123929 Free PMC article.
-
BMP/SMAD Pathway Promotes Neurogenesis of Midbrain Dopaminergic Neurons In Vivo and in Human Induced Pluripotent and Neural Stem Cells.J Neurosci. 2018 Feb 14;38(7):1662-1676. doi: 10.1523/JNEUROSCI.1540-17.2018. Epub 2018 Jan 10. J Neurosci. 2018. PMID: 29321139 Free PMC article.
-
The Neocortical Progenitor Specification Program Is Established through Combined Modulation of SHH and FGF Signaling.J Neurosci. 2020 Sep 2;40(36):6872-6887. doi: 10.1523/JNEUROSCI.2888-19.2020. Epub 2020 Jul 31. J Neurosci. 2020. PMID: 32737167 Free PMC article.
-
Epigenetic regulation of neural stem cell fate during corticogenesis.Int J Dev Neurosci. 2013 Oct;31(6):424-33. doi: 10.1016/j.ijdevneu.2013.02.006. Epub 2013 Mar 4. Int J Dev Neurosci. 2013. PMID: 23466416 Review.
-
Crosstalk of Intercellular Signaling Pathways in the Generation of Midbrain Dopaminergic Neurons In Vivo and from Stem Cells.J Dev Biol. 2019 Jan 15;7(1):3. doi: 10.3390/jdb7010003. J Dev Biol. 2019. PMID: 30650592 Free PMC article. Review.
Cited by
-
Single-cell analyses of X Chromosome inactivation dynamics and pluripotency during differentiation.Genome Res. 2016 Oct;26(10):1342-1354. doi: 10.1101/gr.201954.115. Epub 2016 Aug 2. Genome Res. 2016. PMID: 27486082 Free PMC article.
-
Human neural organoids: Models for developmental neurobiology and disease.Dev Biol. 2021 Oct;478:102-121. doi: 10.1016/j.ydbio.2021.06.012. Epub 2021 Jun 25. Dev Biol. 2021. PMID: 34181916 Free PMC article. Review.
-
Stem cell-derived brainstem mouse astrocytes obtain a neurotoxic phenotype in vitro upon neuroinflammation.J Inflamm (Lond). 2023 Jun 27;20(1):22. doi: 10.1186/s12950-023-00349-8. J Inflamm (Lond). 2023. PMID: 37370141 Free PMC article.
-
Urine-derived induced pluripotent stem cells as a modeling tool for paroxysmal kinesigenic dyskinesia.Biol Open. 2015 Nov 30;4(12):1744-52. doi: 10.1242/bio.013078. Biol Open. 2015. PMID: 26621826 Free PMC article.
-
Covalent growth factor tethering to direct neural stem cell differentiation and self-organization.Acta Biomater. 2017 Apr 15;53:140-151. doi: 10.1016/j.actbio.2017.01.068. Epub 2017 Feb 2. Acta Biomater. 2017. PMID: 28161574 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

