Identification of longitudinally dynamic biomarkers in Alzheimer's disease cerebrospinal fluid by targeted proteomics
- PMID: 24902845
- PMCID: PMC4061120
- DOI: 10.1186/1750-1326-9-22
Identification of longitudinally dynamic biomarkers in Alzheimer's disease cerebrospinal fluid by targeted proteomics
Abstract
Background: Alzheimer's disease (AD) is the leading cause of dementia affecting greater than 26 million people worldwide. Although cerebrospinal fluid (CSF) levels of Aβ42, tau, and p-tau181 are well established as diagnostic biomarkers of AD, there is a need for additional CSF biomarkers of neuronal function that continue to change during disease progression and could be used as pharmacodynamic measures in clinical trials. Multiple proteomic discovery experiments have reported a range of CSF biomarkers that differ between AD and control subjects. These potential biomarkers represent multiple aspects of the disease pathology. The performance of these markers has not been compared with each other, and their performance has not been evaluated longitudinally.
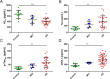
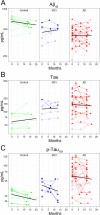
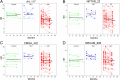
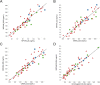
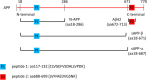
Results: We developed a targeted-proteomic, multiple reaction monitoring (MRM) assay for the absolute quantitation of 39 peptides corresponding to 30 proteins. We evaluated the candidate biomarkers in longitudinal CSF samples collected from aged, cognitively-normal control (n = 10), MCI (n = 5), and AD (n = 45) individuals (age > 60 years). We evaluated each biomarker for diagnostic sensitivity, longitudinal consistency, and compared with CSF Aβ42, tau, and p-tau181. Four of 28 quantifiable CSF proteins were significantly different between aged, cognitively-normal controls and AD subjects including chitinase-3-like protein 1, reproducing published results. Four CSF markers demonstrated significant longitudinal change in AD: Amyloid precursor protein, Neuronal pentraxin receptor, NrCAM and Chromogranin A. Robust correlations were observed within some subgroups of proteins including the potential disease progression markers.
Conclusion: Using a targeted proteomics approach, we confirmed previous findings for a subset of markers, defined longitudinal performance of our panel of markers, and established a flexible proteomics method for robust multiplexed analyses.
Figures

Similar articles
-
Cerebrospinal Fluid Markers of Neurodegeneration and Rates of Brain Atrophy in Early Alzheimer Disease.JAMA Neurol. 2015 Jun;72(6):656-65. doi: 10.1001/jamaneurol.2015.0202. JAMA Neurol. 2015. PMID: 25867677 Free PMC article.
-
Longitudinal Cerebrospinal Fluid Biomarker Changes in Preclinical Alzheimer Disease During Middle Age.JAMA Neurol. 2015 Sep;72(9):1029-42. doi: 10.1001/jamaneurol.2015.1285. JAMA Neurol. 2015. PMID: 26147946 Free PMC article.
-
A targeted proteomic multiplex CSF assay identifies increased malate dehydrogenase and other neurodegenerative biomarkers in individuals with Alzheimer's disease pathology.Transl Psychiatry. 2016 Nov 15;6(11):e952. doi: 10.1038/tp.2016.194. Transl Psychiatry. 2016. PMID: 27845782 Free PMC article.
-
Cerebrospinal fluid proteomics and biological heterogeneity in Alzheimer's disease: A literature review.Crit Rev Clin Lab Sci. 2020 Mar;57(2):86-98. doi: 10.1080/10408363.2019.1670613. Epub 2019 Nov 7. Crit Rev Clin Lab Sci. 2020. PMID: 31694431 Review.
-
Simultaneous analysis of cerebrospinal fluid biomarkers using microsphere-based xMAP multiplex technology for early detection of Alzheimer's disease.Methods. 2012 Apr;56(4):484-93. doi: 10.1016/j.ymeth.2012.03.023. Epub 2012 Apr 6. Methods. 2012. PMID: 22503777 Review.
Cited by
-
In-depth Site-specific Analysis of N-glycoproteome in Human Cerebrospinal Fluid and Glycosylation Landscape Changes in Alzheimer's Disease.Mol Cell Proteomics. 2021;20:100081. doi: 10.1016/j.mcpro.2021.100081. Epub 2021 Apr 20. Mol Cell Proteomics. 2021. PMID: 33862227 Free PMC article.
-
Brain Hydrophobic Peptides Antagonists of Neurotoxic Amyloid β Peptide Monomers/Oligomers-Protein Interactions.Int J Mol Sci. 2023 Sep 8;24(18):13846. doi: 10.3390/ijms241813846. Int J Mol Sci. 2023. PMID: 37762148 Free PMC article. Review.
-
Neurodegeneration, synaptic dysfunction, and gliosis are phenotypic of Alzheimer dementia.Neurology. 2018 Jul 31;91(5):e436-e443. doi: 10.1212/WNL.0000000000005901. Epub 2018 Jun 29. Neurology. 2018. PMID: 29959263 Free PMC article.
-
Neurogranin as a cognitive biomarker in cerebrospinal fluid and blood exosomes for Alzheimer's disease and mild cognitive impairment.Transl Psychiatry. 2020 Apr 29;10(1):125. doi: 10.1038/s41398-020-0801-2. Transl Psychiatry. 2020. PMID: 32350238 Free PMC article.
-
High Resolution Discovery Proteomics Reveals Candidate Disease Progression Markers of Alzheimer's Disease in Human Cerebrospinal Fluid.PLoS One. 2015 Aug 13;10(8):e0135365. doi: 10.1371/journal.pone.0135365. eCollection 2015. PLoS One. 2015. PMID: 26270474 Free PMC article. Clinical Trial.
References
-
- Thies W, Bleiler L. Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9(2):208–245. - PubMed
-
- Sunderland T, Linker G, Mirza N, Putnam KT, Friedman DL, Kimmel LH, Bergeson J, Manetti GJ, Zimmermann M, Tang B, Bartko JJ, Cohen RM. Decreased beta-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 2003;289(16):2094–2103. - PubMed
-
- Clark CM, Xie S, Chittams J, Ewbank D, Peskind E, Galasko D, Morris JC, McKeel DW Jr, Farlow M, Weitlauf SL, Quinn J, Kaye J, Knopman D, Arai H, Doody RS, DeCarli C, Leight S, Lee VM, Trojanowski JQ. Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch Neurol. 2003;60(12):1696–1702. doi: 10.1001/archneur.60.12.1696. - DOI - PubMed
-
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. - DOI - PMC - PubMed
-
- Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of beta-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69(1):98–106. doi: 10.1001/archgenpsychiatry.2011.155. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

