Integrated approaches for analyzing U1-70K cleavage in Alzheimer's disease
- PMID: 24902715
- PMCID: PMC4227550
- DOI: 10.1021/pr5003593
Integrated approaches for analyzing U1-70K cleavage in Alzheimer's disease
Abstract
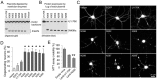
The accumulation of pathologic protein fragments is common in neurodegenerative disorders. We have recently identified in Alzheimer's disease (AD) the aggregation of the U1-70K splicing factor and abnormal RNA processing. Here, we present that U1-70K can be cleaved into an N-terminal truncation (N40K) in ∼50% of AD cases, and the N40K abundance is inversely proportional to the total level of U1-70K. To map the cleavage site, we compared tryptic peptides of N40K and stable isotope labeled U1-70K by liquid chromatography-tandem mass spectrometry (MS), revealing that the proteolysis site is located in a highly repetitive and hydrophilic domain of U1-70K. We then adapted Western blotting to map the cleavage site in two steps: (i) mass spectrometric analysis revealing that U1-70K and N40K share the same N-termini and contain no major modifications; (ii) matching N40K with a series of six recombinant U1-70K truncations to define the cleavage site within a small region (Arg300 ± 6 residues). Finally, N40K expression led to substantial degeneration of rat primary hippocampal neurons. In summary, we combined multiple approaches to identify the U1-70K proteolytic site and found that the N40K fragment might contribute to neuronal toxicity in Alzheimer's disease.
Keywords: Alzheimer’s disease; LC−MS/MS; RNA splicing; U1 snRNP; U1-70K; mass spectrometry; neurodegenerative disease; proteolytic cleavage; proteomics; stable isotope labeling.
Figures

Similar articles
-
Alzheimer's disease-associated U1 snRNP splicing dysfunction causes neuronal hyperexcitability and cognitive impairment.Nat Aging. 2022 Oct;2(10):923-940. doi: 10.1038/s43587-022-00290-0. Epub 2022 Oct 12. Nat Aging. 2022. PMID: 36636325 Free PMC article.
-
U1 small nuclear ribonucleoprotein complex and RNA splicing alterations in Alzheimer's disease.Proc Natl Acad Sci U S A. 2013 Oct 8;110(41):16562-7. doi: 10.1073/pnas.1310249110. Epub 2013 Sep 10. Proc Natl Acad Sci U S A. 2013. PMID: 24023061 Free PMC article.
-
Aggregation properties of the small nuclear ribonucleoprotein U1-70K in Alzheimer disease.J Biol Chem. 2014 Dec 19;289(51):35296-313. doi: 10.1074/jbc.M114.562959. Epub 2014 Oct 29. J Biol Chem. 2014. PMID: 25355317 Free PMC article.
-
RNA-binding proteins with basic-acidic dipeptide (BAD) domains self-assemble and aggregate in Alzheimer's disease.J Biol Chem. 2018 Jul 13;293(28):11047-11066. doi: 10.1074/jbc.RA118.001747. Epub 2018 May 25. J Biol Chem. 2018. PMID: 29802200 Free PMC article.
-
Deep Profiling of the Aggregated Proteome in Alzheimer's Disease: From Pathology to Disease Mechanisms.Proteomes. 2018 Nov 12;6(4):46. doi: 10.3390/proteomes6040046. Proteomes. 2018. PMID: 30424485 Free PMC article. Review.
Cited by
-
Dissecting Detergent-Insoluble Proteome in Alzheimer's Disease by TMTc-Corrected Quantitative Mass Spectrometry.Mol Cell Proteomics. 2023 Aug;22(8):100608. doi: 10.1016/j.mcpro.2023.100608. Epub 2023 Jun 24. Mol Cell Proteomics. 2023. PMID: 37356496 Free PMC article.
-
Sequential Elution Interactome Analysis of the Mind Bomb 1 Ubiquitin Ligase Reveals a Novel Role in Dendritic Spine Outgrowth.Mol Cell Proteomics. 2015 Jul;14(7):1898-910. doi: 10.1074/mcp.M114.045898. Epub 2015 Apr 30. Mol Cell Proteomics. 2015. PMID: 25931508 Free PMC article.
-
Deep Proteome Profiling by Isobaric Labeling, Extensive Liquid Chromatography, Mass Spectrometry, and Software-assisted Quantification.J Vis Exp. 2017 Nov 15;(129):56474. doi: 10.3791/56474. J Vis Exp. 2017. PMID: 29286450 Free PMC article.
-
Non-Coding RNAs in Psychiatric Disorders and Suicidal Behavior.Front Psychiatry. 2020 Sep 15;11:543893. doi: 10.3389/fpsyt.2020.543893. eCollection 2020. Front Psychiatry. 2020. PMID: 33101077 Free PMC article. Review.
-
U1 snRNA over-expression affects neural oscillations and short-term memory deficits in mice.Cogn Neurodyn. 2019 Aug;13(4):313-323. doi: 10.1007/s11571-019-09528-x. Epub 2019 Mar 28. Cogn Neurodyn. 2019. PMID: 31354878 Free PMC article.
References
-
- 2012 Alzheimer’s disease facts and figures. Alzheimer's Dementia 2012, 8 (2), 131–68. - PubMed
-
- Jonsson T.; Stefansson H.; Steinberg S.; Jonsdottir I.; Jonsson P. V.; Snaedal J.; Bjornsson S.; Huttenlocher J.; Levey A. I.; Lah J. J.; Rujescu D.; Hampel H.; Giegling I.; Andreassen O. A.; Engedal K.; Ulstein I.; Djurovic S.; Ibrahim-Verbaas C.; Hofman A.; Ikram M. A.; van Duijn C. M.; Thorsteinsdottir U.; Kong A.; Stefansson K. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013, 3682107–16. - PMC - PubMed
-
- Neumann H.; Daly M. J. Variant TREM2 as risk factor for Alzheimer’s disease. N. Engl. J. Med. 2013, 3682182–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

