Preparation and functional studies of hydroxyethyl chitosan nanoparticles loaded with anti-human death receptor 5 single-chain antibody
- PMID: 24899816
- PMCID: PMC4039402
- DOI: 10.2147/OTT.S59872
Preparation and functional studies of hydroxyethyl chitosan nanoparticles loaded with anti-human death receptor 5 single-chain antibody
Abstract
Objective: To prepare hydroxyethyl chitosan nanoparticles loaded with anti-human death receptor 5 single-chain antibody, and study their characteristics, functions, and mechanisms of action.
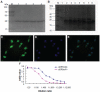
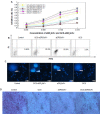
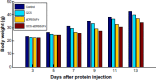
Materials and methods: The anti-human death receptor 5 single-chain antibody was constructed and expressed. Protein-loaded hydroxyethyl chitosan nanoparticles were prepared, and their size, morphology, particle-size distribution and surface zeta potential were measured by scanning electron microscopy and laser particle-size analysis. Mouse H22 hepatocellular carcinoma cells were cultured, and growth inhibition was examined using the CellTiter-Blue cell-viability assay. Flow cytometry and Hoechst 33342 were employed to measure cell apoptosis. Kunming mice with H22 tumor models were treated with protein-loaded hydroxyethyl chitosan nanoparticles, and their body weight and tumor size were measured, while hematoxylin and eosin staining was used to detect antitumor effects in vivo and side effects from tumors.
Results: The protein-loaded hydroxyethyl chitosan nanoparticles had good stability; the zeta potential was -24.2±0.205, and the dispersion index was 0.203. The inhibition of the protein-loaded hydroxyethyl chitosan nanoparticles on H22 growth was both time- and dose-dependent. Increased expressions of active caspase 8, active caspase 3, and BAX were detected following treatment. The average weight gain, tumor weight, and mean tumor volume of the protein and protein-loaded hydroxyethyl chitosan nanoparticle groups were significantly different (P<0.05) compared with the phosphate-buffered saline group.
Conclusion: The protein-loaded hydroxyethyl chitosan nanoparticles effectively suppressed tumor growth, indicating that nanotechnology has the potential for broad application in cancer therapy.
Keywords: DR5; GCS-aDR5ScFv; H22; anticancer effect.
Figures


Similar articles
-
Preparation of carboplatin-Fe@C-loaded chitosan nanoparticles and study on hyperthermia combined with pharmacotherapy for liver cancer.Int J Hyperthermia. 2009 Aug;25(5):383-91. doi: 10.1080/02656730902834949. Int J Hyperthermia. 2009. PMID: 19391033
-
Galactosylated chitosan/5-fluorouracil nanoparticles inhibit mouse hepatic cancer growth and its side effects.World J Gastroenterol. 2012 Nov 14;18(42):6076-87. doi: 10.3748/wjg.v18.i42.6076. World J Gastroenterol. 2012. PMID: 23155336 Free PMC article.
-
Cytotoxicity and apoptotic effects of tea polyphenol-loaded chitosan nanoparticles on human hepatoma HepG2 cells.Mater Sci Eng C Mater Biol Appl. 2014 Mar 1;36:7-13. doi: 10.1016/j.msec.2013.11.039. Epub 2013 Dec 5. Mater Sci Eng C Mater Biol Appl. 2014. PMID: 24433880
-
Preparation and Nanoencapsulation of Lectin from Lepidium sativum on Chitosan-Tripolyphosphate Nanoparticle and Their Cytotoxicity against Hepatocellular Carcinoma Cells (HepG2).Biomed Res Int. 2020 Oct 22;2020:7251346. doi: 10.1155/2020/7251346. eCollection 2020. Biomed Res Int. 2020. PMID: 33145357 Free PMC article.
-
Phenylboronic Acid-Mediated Tumor Targeting of Chitosan Nanoparticles.Theranostics. 2016 Jun 15;6(9):1378-92. doi: 10.7150/thno.15156. eCollection 2016. Theranostics. 2016. PMID: 27375786 Free PMC article.
Cited by
-
Anti-Fn14 Antibody-Conjugated Nanoparticles Display Membrane TWEAK-Like Agonism.Pharmaceutics. 2021 Jul 13;13(7):1072. doi: 10.3390/pharmaceutics13071072. Pharmaceutics. 2021. PMID: 34371763 Free PMC article.
-
The pro-apoptotic effects of TIPE2 on AA rat fibroblast-like synoviocytes via regulation of the DR5-caspase-NF-κB pathway in vitro.Onco Targets Ther. 2016 Feb 29;9:993-1000. doi: 10.2147/OTT.S92907. eCollection 2016. Onco Targets Ther. 2016. PMID: 27013892 Free PMC article.
-
Clinical significance of TIPE expression in gastric carcinoma.Onco Targets Ther. 2016 Jul 25;9:4473-81. doi: 10.2147/OTT.S100593. eCollection 2016. Onco Targets Ther. 2016. PMID: 27524904 Free PMC article.
-
Recent Advances in the Development of Nanodelivery Systems Targeting the TRAIL Death Receptor Pathway.Pharmaceutics. 2023 Feb 3;15(2):515. doi: 10.3390/pharmaceutics15020515. Pharmaceutics. 2023. PMID: 36839837 Free PMC article. Review.
References
-
- Marrero JA. Hepatocellular carcinoma. Curr Opin Gastroenterol. 2006;22:248–253. - PubMed
-
- Motola-Kuba D, Zamora-Valdés D, Uribe M, Méndez-Sánchez N. Hepatocellular carcinoma. An overview. Ann Hepatol. 2006;5:16–24. - PubMed
-
- Vasir JK, Red MK, Labhasetwar VD. Nanosystems in drug targeting: opportunities and challenges. Curr Nanosci. 2005;1:47–64.
-
- French LE, Tschopp J. Protein-based therapeutic approaches targeting death receptors. Cell Death Differ. 2003;10:117–123. - PubMed
-
- Sheridan JP, Marsters SA, Pitti RM, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

