The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes
- PMID: 24899308
- PMCID: PMC4489847
- DOI: 10.1038/nature13478
The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes
Abstract
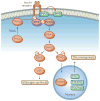
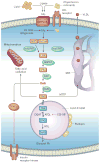
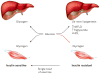
Non-alcoholic fatty liver disease and its downstream sequelae, hepatic insulin resistance and type 2 diabetes, are rapidly growing epidemics, which lead to increased morbidity and mortality rates, and soaring health-care costs. Developing interventions requires a comprehensive understanding of the mechanisms by which excess hepatic lipid develops and causes hepatic insulin resistance and type 2 diabetes. Proposed mechanisms implicate various lipid species, inflammatory signalling and other cellular modifications. Studies in mice and humans have elucidated a key role for hepatic diacylglycerol activation of protein kinase Cε in triggering hepatic insulin resistance. Therapeutic approaches based on this mechanism could alleviate the related epidemics of non-alcoholic fatty liver disease and type 2 diabetes.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Diacylglycerol activation of protein kinase Cε and hepatic insulin resistance.Cell Metab. 2012 May 2;15(5):574-84. doi: 10.1016/j.cmet.2012.03.005. Cell Metab. 2012. PMID: 22560210 Free PMC article. Review.
-
Lipid-induced insulin resistance: unravelling the mechanism.Lancet. 2010 Jun 26;375(9733):2267-77. doi: 10.1016/S0140-6736(10)60408-4. Lancet. 2010. PMID: 20609972 Free PMC article. Review.
-
Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes.Hepatology. 2014 Feb;59(2):713-23. doi: 10.1002/hep.26672. Hepatology. 2014. PMID: 23929732 Free PMC article. Review.
-
Ectopic lipid accumulation: A potential cause for metabolic disturbances and a contributor to the alteration of kidney function.Biochimie. 2013 Nov;95(11):1971-9. doi: 10.1016/j.biochi.2013.07.017. Epub 2013 Jul 27. Biochimie. 2013. PMID: 23896376 Review.
-
Mechanisms for insulin resistance: common threads and missing links.Cell. 2012 Mar 2;148(5):852-71. doi: 10.1016/j.cell.2012.02.017. Cell. 2012. PMID: 22385956 Free PMC article. Review.
Cited by
-
Lipid profiling of the therapeutic effects of berberine in patients with nonalcoholic fatty liver disease.J Transl Med. 2016 Sep 15;14:266. doi: 10.1186/s12967-016-0982-x. J Transl Med. 2016. PMID: 27629750 Free PMC article. Clinical Trial.
-
Adipose tissue macrophages exert systemic metabolic control by manipulating local iron concentrations.Nat Metab. 2022 Nov;4(11):1474-1494. doi: 10.1038/s42255-022-00664-z. Epub 2022 Nov 3. Nat Metab. 2022. PMID: 36329217 Free PMC article.
-
Nonalcoholic Fatty Liver Disease (NAFLD). Mitochondria as Players and Targets of Therapies?Int J Mol Sci. 2021 May 20;22(10):5375. doi: 10.3390/ijms22105375. Int J Mol Sci. 2021. PMID: 34065331 Free PMC article. Review.
-
Non-Alcoholic Fatty Liver Disease in Obese Youth With Insulin Resistance and Type 2 Diabetes.Front Endocrinol (Lausanne). 2021 Apr 6;12:639548. doi: 10.3389/fendo.2021.639548. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33889132 Free PMC article. Review.
-
Reduced mitochondrial mass and function add to age-related susceptibility toward diet-induced fatty liver in C57BL/6J mice.Physiol Rep. 2016 Oct;4(19):e12988. doi: 10.14814/phy2.12988. Physiol Rep. 2016. PMID: 27694529 Free PMC article.
References
-
- Browning JD, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. - PubMed
-
- Smits MM, Ioannou GN, Boyko EJ, Utzschneider KM. Non-alcoholic fatty liver disease as an independent manifestation of the metabolic syndrome: results of a US national survey in three ethnic groups. J Gastroenterol Hepatol. 2013;28:664–670. - PubMed
-
- Fan JG, et al. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol. 2005;43:508–514. - PubMed
-
- Amarapurkar DN, et al. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J Gastroenterol Hepatol. 2007;22:788–793. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 RR024139/RR/NCRR NIH HHS/United States
- R01 DK-40936/DK/NIDDK NIH HHS/United States
- R24 DK-085836/DK/NIDDK NIH HHS/United States
- I01 BX000901/BX/BLRD VA/United States
- P30 DK-45735/DK/NIDDK NIH HHS/United States
- R01 DK040936/DK/NIDDK NIH HHS/United States
- T32-DK101019/DK/NIDDK NIH HHS/United States
- R01 AG023686/AG/NIA NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- U24 DK-059635/DK/NIDDK NIH HHS/United States
- R01 DK-49230/DK/NIDDK NIH HHS/United States
- P30 DK034989/DK/NIDDK NIH HHS/United States
- R01 AG-23686/AG/NIA NIH HHS/United States
- T32 DK101019/DK/NIDDK NIH HHS/United States
- R01 DK049230/DK/NIDDK NIH HHS/United States
- R13 DK085836/DK/NIDDK NIH HHS/United States
- UL1 RR-024139/RR/NCRR NIH HHS/United States
- U24 DK059635/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

