Microscopic inner retinal hyper-reflective phenotypes in retinal and neurologic disease
- PMID: 24894394
- PMCID: PMC4078949
- DOI: 10.1167/iovs.14-14668
Microscopic inner retinal hyper-reflective phenotypes in retinal and neurologic disease
Abstract
Purpose: We surveyed inner retinal microscopic features in retinal and neurologic disease using a reflectance confocal adaptive optics scanning light ophthalmoscope (AOSLO).
Methods: Inner retinal images from 101 subjects affected by one of 38 retinal or neurologic conditions and 11 subjects with no known eye disease were examined for the presence of hyper-reflective features other than vasculature, retinal nerve fiber layer, and foveal pit reflex. The hyper-reflective features in the AOSLO images were grouped based on size, location, and subjective texture. Clinical imaging, including optical coherence tomography (OCT), scanning laser ophthalmoscopy, and fundus photography was analyzed for comparison.
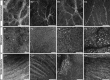
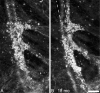
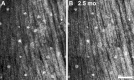
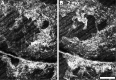
Results: Seven categories of hyper-reflective inner retinal structures were identified, namely punctate reflectivity, nummular (disc-shaped) reflectivity, granular membrane, waxy membrane, vessel-associated membrane, microcysts, and striate reflectivity. Punctate and nummular reflectivity also was found commonly in normal volunteers, but the features in the remaining five categories were found only in subjects with retinal or neurologic disease. Some of the features were found to change substantially between follow up imaging months apart.
Conclusions: Confocal reflectance AOSLO imaging revealed a diverse spectrum of normal and pathologic hyper-reflective inner and epiretinal features, some of which were previously unreported. Notably, these features were not disease-specific, suggesting that they might correspond to common mechanisms of degeneration or repair in pathologic states. Although prospective studies with larger and better characterized populations, along with imaging of more extensive retinal areas are needed, the hyper-reflective structures reported here could be used as disease biomarkers, provided their specificity is studied further.
Keywords: adaptive optics; inner retina; neuro-ophthalmology; ophthalmoscopy; retinal disease.
Copyright 2014 The Association for Research in Vision and Ophthalmology, Inc.
Figures










Similar articles
-
Characterization of Inner Retinal Hyperreflective Alterations in Early Cognitive Impairment on Adaptive Optics Scanning Laser Ophthalmoscopy.Invest Ophthalmol Vis Sci. 2019 Aug 1;60(10):3527-3536. doi: 10.1167/iovs.19-27135. Invest Ophthalmol Vis Sci. 2019. PMID: 31412112 Free PMC article.
-
Adaptive optics scanning laser ophthalmoscopy in a heterogenous cohort with Stargardt disease.Sci Rep. 2024 Oct 9;14(1):23629. doi: 10.1038/s41598-024-74088-y. Sci Rep. 2024. PMID: 39384610 Free PMC article.
-
The fundus photo has met its match: optical coherence tomography and adaptive optics ophthalmoscopy are here to stay.Ophthalmic Physiol Opt. 2016 May;36(3):218-39. doi: 10.1111/opo.12289. Ophthalmic Physiol Opt. 2016. PMID: 27112222 Free PMC article. Review.
-
Photoreceptor perturbation around subretinal drusenoid deposits as revealed by adaptive optics scanning laser ophthalmoscopy.Am J Ophthalmol. 2014 Sep;158(3):584-96.e1. doi: 10.1016/j.ajo.2014.05.038. Epub 2014 Jun 5. Am J Ophthalmol. 2014. PMID: 24907433 Free PMC article.
-
[Applications of optical coherence tomography (OCT) in neuro-ophthalmology].Klin Monbl Augenheilkd. 2013 Nov;230(11):1097-105. doi: 10.1055/s-0033-1350786. Epub 2013 Sep 24. Klin Monbl Augenheilkd. 2013. PMID: 24065512 Review. German.
Cited by
-
Discovery and clinical translation of novel glaucoma biomarkers.Prog Retin Eye Res. 2021 Jan;80:100875. doi: 10.1016/j.preteyeres.2020.100875. Epub 2020 Jul 10. Prog Retin Eye Res. 2021. PMID: 32659431 Free PMC article. Review.
-
Application of novel non-invasive ophthalmic imaging to visualize peripapillary wrinkles, retinal folds and peripapillary hyperreflective ovoid mass-like structures associated with elevated intracranial pressure.Front Neurol. 2024 Jun 18;15:1383210. doi: 10.3389/fneur.2024.1383210. eCollection 2024. Front Neurol. 2024. PMID: 38957348 Free PMC article.
-
Characterization of Inner Retinal Hyperreflective Alterations in Early Cognitive Impairment on Adaptive Optics Scanning Laser Ophthalmoscopy.Invest Ophthalmol Vis Sci. 2019 Aug 1;60(10):3527-3536. doi: 10.1167/iovs.19-27135. Invest Ophthalmol Vis Sci. 2019. PMID: 31412112 Free PMC article.
-
Progression of Local Glaucomatous Damage Near Fixation as Seen with Adaptive Optics Imaging.Transl Vis Sci Technol. 2017 Jul 12;6(4):6. doi: 10.1167/tvst.6.4.6. eCollection 2017 Jul. Transl Vis Sci Technol. 2017. PMID: 28713646 Free PMC article.
-
Promises and pitfalls of evaluating photoreceptor-based retinal disease with adaptive optics scanning light ophthalmoscopy (AOSLO).Prog Retin Eye Res. 2021 Jul;83:100920. doi: 10.1016/j.preteyeres.2020.100920. Epub 2020 Nov 6. Prog Retin Eye Res. 2021. PMID: 33161127 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

