Minireview: Gut microbiota: the neglected endocrine organ
- PMID: 24892638
- PMCID: PMC5414803
- DOI: 10.1210/me.2014-1108
Minireview: Gut microbiota: the neglected endocrine organ
Abstract
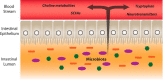
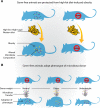
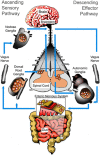
The concept that the gut microbiota serves as a virtual endocrine organ arises from a number of important observations. Evidence for a direct role arises from its metabolic capacity to produce and regulate multiple compounds that reach the circulation and act to influence the function of distal organs and systems. For example, metabolism of carbohydrates results in the production of short-chain fatty acids, such as butyrate and propionate, which provide an important source of nutrients as well as regulatory control of the host digestive system. This influence over host metabolism is also seen in the ability of the prebiotic inulin to influence production of relevant hormones such as glucagon-like peptide-1, peptide YY, ghrelin, and leptin. Moreover, the probiotic Lactobacillus rhamnosus PL60, which produces conjugated linoleic acid, has been shown to reduce body-weight gain and white adipose tissue without effects on food intake. Manipulating the microbial composition of the gastrointestinal tract modulates plasma concentrations of tryptophan, an essential amino acid and precursor to serotonin, a key neurotransmitter within both the enteric and central nervous systems. Indirectly and through as yet unknown mechanisms, the gut microbiota exerts control over the hypothalamic-pituitary-adrenal axis. This is clear from studies on animals raised in a germ-free environment, who show exaggerated responses to psychological stress, which normalizes after monocolonization by certain bacterial species including Bifidobacterium infantis. It is tempting to speculate that therapeutic targeting of the gut microbiota may be useful in treating stress-related disorders and metabolic diseases.
Figures
Similar articles
-
The gut microbiome as a virtual endocrine organ with implications for farm and domestic animal endocrinology.Domest Anim Endocrinol. 2016 Jul;56 Suppl:S44-55. doi: 10.1016/j.domaniend.2016.05.003. Domest Anim Endocrinol. 2016. PMID: 27345323 Review.
-
Gut/brain axis and the microbiota.J Clin Invest. 2015 Mar 2;125(3):926-38. doi: 10.1172/JCI76304. Epub 2015 Feb 17. J Clin Invest. 2015. PMID: 25689247 Free PMC article. Review.
-
The gut microbiome and the brain.J Med Food. 2014 Dec;17(12):1261-72. doi: 10.1089/jmf.2014.7000. J Med Food. 2014. PMID: 25402818 Free PMC article. Review.
-
Serotonin, tryptophan metabolism and the brain-gut-microbiome axis.Behav Brain Res. 2015 Jan 15;277:32-48. doi: 10.1016/j.bbr.2014.07.027. Epub 2014 Jul 29. Behav Brain Res. 2015. PMID: 25078296 Review.
-
Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes.J Int Soc Sports Nutr. 2016 Nov 24;13:43. doi: 10.1186/s12970-016-0155-6. eCollection 2016. J Int Soc Sports Nutr. 2016. PMID: 27924137 Free PMC article. Review.
Cited by
-
The Gut Microbiota Modulates Neuroinflammation in Alzheimer's Disease: Elucidating Crucial Factors and Mechanistic Underpinnings.CNS Neurosci Ther. 2024 Oct;30(10):e70091. doi: 10.1111/cns.70091. CNS Neurosci Ther. 2024. PMID: 39460538 Free PMC article. Review.
-
The Microbiota-Gut-Brain Axis and Neurological Disorders: A Comprehensive Review.Life (Basel). 2024 Sep 26;14(10):1234. doi: 10.3390/life14101234. Life (Basel). 2024. PMID: 39459534 Free PMC article. Review.
-
Necrotizing Enterocolitis and Neurodevelopmental Impairments: Microbiome, Gut, and Brain Entanglements.Biomolecules. 2024 Oct 4;14(10):1254. doi: 10.3390/biom14101254. Biomolecules. 2024. PMID: 39456187 Free PMC article. Review.
-
Gut Microbiota Mediates Neuroinflammation in Alzheimer's Disease: Unraveling Key Factors and Mechanistic Insights.Mol Neurobiol. 2024 Sep 25. doi: 10.1007/s12035-024-04513-w. Online ahead of print. Mol Neurobiol. 2024. PMID: 39317889 Review.
-
Identification of proteotoxic and proteoprotective bacteria that non-specifically affect proteins associated with neurodegenerative diseases.iScience. 2024 Aug 27;27(9):110828. doi: 10.1016/j.isci.2024.110828. eCollection 2024 Sep 20. iScience. 2024. PMID: 39310761 Free PMC article.
References
-
- Trompette A, Gollwitzer ES, Yadava K, et al. . Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. - PubMed
-
- Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain Behav Immun. 2010;24:9–16. - PubMed
-
- Evans JM, Morris LS, Marchesi JR. The gut microbiome: the role of a virtual organ in the endocrinology of the host. J Endocrinol. 2013;218:R37–R47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

