Direct transcriptional repression of Zfp423 by Zfp521 mediates a bone morphogenic protein-dependent osteoblast versus adipocyte lineage commitment switch
- PMID: 24891617
- PMCID: PMC4135594
- DOI: 10.1128/MCB.00185-14
Direct transcriptional repression of Zfp423 by Zfp521 mediates a bone morphogenic protein-dependent osteoblast versus adipocyte lineage commitment switch
Abstract
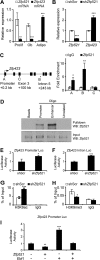
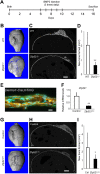
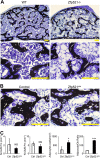
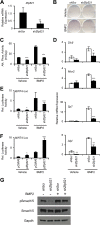
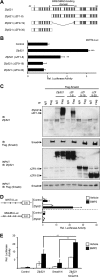
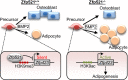
Osteoblasts and adipocytes arise from a common mesenchymal precursor cell. The cell fate decision of a mesenchymal precursor cell is under the influence of molecular cues and signaling pathways that lead to the activation or repression of lineage-specific transcription factors. The molecular mechanisms determining osteoblast versus adipocyte lineage specificity in response to bone morphogenic protein (BMP) remain unclear. In this study, we describe the mechanism through which Zfp521 (ZNF521), a regulator of lineage progression in multiple immature cell populations, regulates lineage specification of mesenchymal progenitor cells during BMP-induced differentiation events. In vivo deletion or in vitro knockdown of Zfp521 in mesenchymal precursors resulted in increased expression of the adipocyte determinant factor Zfp423 (ZNF423). This was concurrent with the loss of histone H3K9 methylation and an increase in histone H3K9 acetylation at the Zfp423 promoter, which together are indicative of decreased gene repression. Indeed, we found that Zfp521 occupies and represses the promoter and intronic enhancer regions of Zfp423. Accordingly, conditional deletion of Zfp521 inhibited heterotopic bone formation in response to local injection of BMP2. In contrast, marrow adiposity within BMP2-induced bone was markedly enhanced in Zfp521-deficient mice, suggesting that precursor cells lacking Zfp521 differentiate preferentially into adipocytes instead of osteoblasts in response to BMP2. Consistent with a cell-autonomous role of Zfp521 in mesenchymal precursors, knockdown of Zfp521 in stromal cells prevented BMP2-induced osteoblast marker expression and simultaneously enhanced lipid accumulation and expression of adipocyte-related genes. Taken together, the data suggest that Zfp521 is a cell fate switch critical for BMP-induced osteoblast commitment and identify Zfp521 as the intrinsic repressor of Zfp423 and hence of adipocyte commitment during BMP-induced mesenchymal precursor differentiation.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Epigenetic modifications of the Zfp/ZNF423 gene control murine adipogenic commitment and are dysregulated in human hypertrophic obesity.Diabetologia. 2018 Feb;61(2):369-380. doi: 10.1007/s00125-017-4471-4. Epub 2017 Oct 24. Diabetologia. 2018. PMID: 29067487 Free PMC article.
-
Regulation of early adipose commitment by Zfp521.PLoS Biol. 2012;10(11):e1001433. doi: 10.1371/journal.pbio.1001433. Epub 2012 Nov 27. PLoS Biol. 2012. PMID: 23209378 Free PMC article.
-
Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages.J Cell Biol. 1998 Jul 13;142(1):295-305. doi: 10.1083/jcb.142.1.295. J Cell Biol. 1998. PMID: 9660882 Free PMC article.
-
Zinc finger protein 521, a new player in bone formation.Ann N Y Acad Sci. 2010 Mar;1192:32-7. doi: 10.1111/j.1749-6632.2009.05347.x. Ann N Y Acad Sci. 2010. PMID: 20392215 Review.
-
PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells.Curr Stem Cell Res Ther. 2016;11(3):216-25. doi: 10.2174/1574888x10666150519093429. Curr Stem Cell Res Ther. 2016. PMID: 25986621 Review.
Cited by
-
Transcriptional regulation of the proto-oncogene Zfp521 by SPI1 (PU.1) and HOXC13.Genesis. 2016 Oct;54(10):519-533. doi: 10.1002/dvg.22963. Epub 2016 Aug 29. Genesis. 2016. PMID: 27506447 Free PMC article.
-
Identification of novel variants associated with osteoporosis, type 2 diabetes and potentially pleiotropic loci using pleiotropic cFDR method.Bone. 2018 Dec;117:6-14. doi: 10.1016/j.bone.2018.08.020. Epub 2018 Aug 30. Bone. 2018. PMID: 30172742 Free PMC article.
-
Zfp423 Regulates Skeletal Muscle Regeneration and Proliferation.Mol Cell Biol. 2019 Apr 2;39(8):e00447-18. doi: 10.1128/MCB.00447-18. Print 2019 Apr 15. Mol Cell Biol. 2019. PMID: 30692273 Free PMC article.
-
Zinc Finger Protein 521 Regulates Early Hematopoiesis through Cell-Extrinsic Mechanisms in the Bone Marrow Microenvironment.Mol Cell Biol. 2018 Aug 15;38(17):e00603-17. doi: 10.1128/MCB.00603-17. Print 2018 Sep 1. Mol Cell Biol. 2018. PMID: 29915154 Free PMC article.
-
bta-miR-23a involves in adipogenesis of progenitor cells derived from fetal bovine skeletal muscle.Sci Rep. 2017 Mar 3;7:43716. doi: 10.1038/srep43716. Sci Rep. 2017. PMID: 28255176 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
