Host cell autophagy promotes BK virus infection
- PMID: 24889228
- PMCID: PMC7112032
- DOI: 10.1016/j.virol.2014.03.009
Host cell autophagy promotes BK virus infection
Abstract
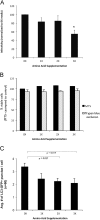
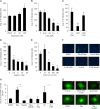
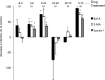
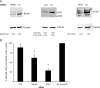
Autophagy is important for a variety for virus life cycles. We sought to determine the role of autophagy in human BK polyomavirus (BKPyV) infection. The addition excess amino acids during viral infection reduced BKPyV infection. Perturbing autophagy levels using inhibitors, 3-MA, bafilomycin A1, and spautin-1, also reduced infection, while rapamycin treatment of host cells increased infection. siRNA knockdown of autophagy genes, ATG7 and Beclin-1, corresponded to a decrease in BKPyV infection. BKPyV infection not only correlated with autophagosome formation, but also virus particles localized to autophagy-specific compartments early in infection. These data support a novel role for autophagy in the promotion of BKPyV infection.
Keywords: Autophagosome; Autophagy; BK; BKPyV; BKV; Nephropathy; Polyomavirus.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Inhibition of BK virus replication in human kidney cells by BK virus large tumor antigen-specific shRNA delivered by JC virus-like particles.Antiviral Res. 2014 Mar;103:25-31. doi: 10.1016/j.antiviral.2013.12.013. Epub 2014 Jan 7. Antiviral Res. 2014. PMID: 24406668
-
Roles of ATM and ATR-mediated DNA damage responses during lytic BK polyomavirus infection.PLoS Pathog. 2012;8(8):e1002898. doi: 10.1371/journal.ppat.1002898. Epub 2012 Aug 30. PLoS Pathog. 2012. PMID: 22952448 Free PMC article.
-
BK Polyomavirus Activates the DNA Damage Response To Prolong S Phase.J Virol. 2019 Jun 28;93(14):e00130-19. doi: 10.1128/JVI.00130-19. Print 2019 Jul 15. J Virol. 2019. PMID: 31043526 Free PMC article.
-
The human polyomavirus BK (BKPyV): virological background and clinical implications.APMIS. 2013 Aug;121(8):728-45. doi: 10.1111/apm.12134. Epub 2013 Jun 19. APMIS. 2013. PMID: 23782063 Review.
-
BK polyomavirus-specific antibody and T-cell responses in kidney transplantation: update.Curr Opin Infect Dis. 2019 Dec;32(6):575-583. doi: 10.1097/QCO.0000000000000602. Curr Opin Infect Dis. 2019. PMID: 31567736 Review.
Cited by
-
Regulation of Autophagy in Cells Infected With Oncogenic Human Viruses and Its Impact on Cancer Development.Front Cell Dev Biol. 2020 Feb 28;8:47. doi: 10.3389/fcell.2020.00047. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32181249 Free PMC article. Review.
-
The endoplasmic reticulum membrane J protein C18 executes a distinct role in promoting simian virus 40 membrane penetration.J Virol. 2015 Apr;89(8):4058-68. doi: 10.1128/JVI.03574-14. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631089 Free PMC article.
-
Interactions between Autophagy and DNA Viruses.Viruses. 2019 Aug 23;11(9):776. doi: 10.3390/v11090776. Viruses. 2019. PMID: 31450758 Free PMC article. Review.
-
SV40 Hijacks Cellular Transport, Membrane Penetration, and Disassembly Machineries to Promote Infection.Viruses. 2019 Oct 5;11(10):917. doi: 10.3390/v11100917. Viruses. 2019. PMID: 31590347 Free PMC article. Review.
-
Biology of the BKPyV: An Update.Viruses. 2017 Nov 3;9(11):327. doi: 10.3390/v9110327. Viruses. 2017. PMID: 29099746 Free PMC article. Review.
References
-
- Blommaart E.F., Krause U., Schellens J.P., Vreeling-Sindelarova H., Meijer A.J. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur. J. Biochem. 1997;243(1-2):240–246. - PubMed
-
- Chua C.E., Tang B.L. Linking membrane dynamics and trafficking to autophagy and the unfolded protein response. J. Cell Physiol. 2013;228(8):1638–1640. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

